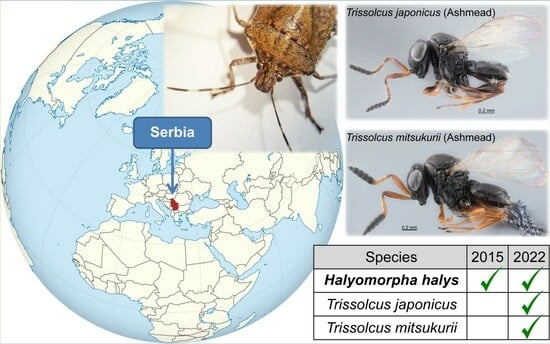
The First Records of Trissolcus japonicus (Ashmead) and Trissolcus mitsukurii (Ashmead) (Hymenoptera, Scelionidae), Alien Egg Parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) in Serbia
Abstract
:Simple Summary
Abstract
1. Introduction

2. Materials and Methods
2.1. Field Collection and Laboratory Rearing of Stink Bug Egg Masses
2.2. Species Identification
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPPO. Halyomorpha halys (HALYHA) [World Distribution] EPPO Global Database. Available online: https://gd.eppo.int/taxon/HALYHA/distribution (accessed on 14 November 2022).
- Bergmann, E.J.; Venugopal, P.D.; Martinson, H.M.; Raupp, M.J.; Shrewsbury, P.M. Host Plant Use by the Invasive Halyomorpha halys (Stål) on Woody Ornamental Trees and Shrubs. PLoS ONE 2016, 11, e0149975. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, T.P.; Kamminga, K. Review of the Chemical Control Research on Halyomorpha halys in the USA. J. Pest Sci. 2017, 90, 1021–1031. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Yao, Y.X.; Qiu, L.F.; Li, Z.X. A New Species of Trissolcus (Hymenoptera: Scelionidae) Parasitizing Eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with Comments on Its Biology. Ann. Entomol. Soc. Am. 2009, 102, 39–47. [Google Scholar] [CrossRef]
- Lee, D.H. Current Status of Research Progress on the Biology and Management of Halyomorpha halys (Hemiptera: Pentatomidae) as an Invasive Species. Appl. Entomol. Zool. 2015, 50, 277–290. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhang, F.; Gariepy, T.D.; Mason, P.G.; Gillespie, D.; Talamas, E.J.; Haye, T. Seasonal Parasitism and Host Specificity of Trissolcus japonicus in Northern China. J. Pest Sci. 2017, 90, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Abram, P.K.; Hoelmer, K.A.; Acebes-Doria, A.L.; Andrews, H.; Beers, E.H.; Bergh, J.C.; Bessin, R.; Biddinger, D.; Botch, P.; Buffington, M.L.; et al. Indigenous Arthropod Natural Enemies of the Invasive Brown Marmorated Stink Bug in North America and Europe. J. Pest Sci. 2017, 90, 1009–1020. [Google Scholar] [CrossRef]
- Costi, E.; Haye, T.; Maistrello, L. Surveying Native Egg Parasitoids and Predators of the Invasive Halyomorpha halys in Northern Italy. J. Appl. Entomol. 2019, 143, 299–307. [Google Scholar] [CrossRef]
- Dieckhoff, C.; Tatman, K.M.; Hoelmer, K.A. Natural Biological Control of Halyomorpha halys by Native Egg Parasitoids: A Multi-Year Survey in Northern Delaware. J. Pest Sci. 2017, 90, 1143–1158. [Google Scholar] [CrossRef]
- Fusu, L.; Andreadis, S.S. Ooencyrtus mirus (Hymenoptera, Encyrtidae), Discovered in Europe Parasitizing Eggs of Halyomorpha halys (Hemiptera, Pentatomidae). J. Hymenopt. Res. 2023, 96, 1045–1060. [Google Scholar] [CrossRef]
- Haye, T.; Fischer, S.; Zhang, J.P.; Gariepy, T.D. Can Native Egg Parasitoids Adopt the Invasive Brown Marmorated Stink Bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? J. Pest Sci. 2015, 88, 693–705. [Google Scholar] [CrossRef]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Foti, M.C.; Peri, E.; Maistrello, L.; Haye, T.; Conti, E. Native Egg Parasitoids Recorded from the Invasive Halyomorpha halys Successfully Exploit Volatiles Emitted by the Plant–Herbivore Complex. J Pest Sci 2017, 90, 1087–1095. [Google Scholar] [CrossRef]
- Stahl, J.M.; Babendreier, D.; Haye, T. Using the Egg Parasitoid Anastatus bifasciatus against the Invasive Brown Marmorated Stink Bug in Europe: Can Non-Target Effects Be Ruled Out? J. Pest Sci. 2018, 91, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Tillman, G.; Toews, M.D.; Blaauw, B.R.; Sial, A.A.; Cottrell, T.E.; Talamas, E.J.; Buntin, D.G.; Joseph, S.V.; Balusu, R.R.; Fadamiro, H.; et al. Parasitism and Predation of Sentinel Eggs of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in the Southeastern US. Biol. Control 2020, 145, 104247. [Google Scholar] [CrossRef]
- Moraglio, S.T.; Tortorici, F.; Visentin, S.; Pansa, M.G.; Tavella, L. Trissolcus kozlovi in North Italy: Host Specificity and Augmentative Releases against Halyomorpha halys in Hazelnut Orchards. Insects 2021, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.; Tortorici, F.; Pontini, M.; Bon, M.C.; Hoelmer, K.A.; Marazzi, C.; Tavella, L.; Haye, T. First Discovery of Adventive Populations of Trissolcus japonicus in Europe. J. Pest Sci. 2019, 92, 371–379. [Google Scholar] [CrossRef]
- Stahl, J.; Babendreier, D.; Marazzi, C.; Caruso, S.; Costi, E.; Maistrello, L.; Haye, T. Can Anastatus bifasciatus Be Used for Augmentative Biological Control of the Brown Marmorated Stink Bug in Fruit Orchards? Insects 2019, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Zapponi, L.; Tortorici, F.; Anfora, G.; Bardella, S.; Bariselli, M.; Benvenuto, L.; Bernardinelli, I.; Butturini, A.; Caruso, S.; Colla, R.; et al. Assessing the Distribution of Exotic Egg Parasitoids of Halyomorpha halys in Europe with a Large-Scale Monitoring Program. Insects 2021, 12, 316. [Google Scholar] [CrossRef]
- Herlihy, M.V.; Talamas, E.J.; Weber, D.C. Attack and Success of Native and Exotic Parasitoids on Eggs of Halyomorpha halys in Three Maryland Habitats. PLoS ONE 2016, 11, e0150275. [Google Scholar] [CrossRef]
- Sabbatini Peverieri, G.; Dieckhoff, C.; Giovannini, L.; Marianelli, L.; Roversi, P.F.; Hoelmer, K.A. Rearing Trissolcus japonicus and Trissolcus mitsukurii for Biological Control of Halyomorpha halys. Insects 2020, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Costi, E.; Di Bella, E.; Iotti, D.; Maistrello, L. Biocontrol Implications of Multiparasitism by Trissolcus mitsukurii and Trissolcus japonicus on the Invasive Brown Marmorated Stink Bug. Entomol. Exp. Appl. 2022, 170, 772–781. [Google Scholar] [CrossRef]
- Giovannini, L.; Sabbatini Peverieri, G.; Marianelli, L.; Rondoni, G.; Conti, E.; Roversi, P.F. Physiological Host Range of Trissolcus mitsukurii, a Candidate Biological Control Agent of Halyomorpha halys in Europe. J. Pest Sci. 2022, 95, 605–618. [Google Scholar] [CrossRef]
- Giovannini, L.; Sabbatini-Peverieri, G.; Simoni, S.; Cervo, R.; Alan Hoelmer, K.; Federico Roversi, P. Interspecific Competition between Trissolcus japonicus and Trissolcus mitsukurii, Two Promising Candidates for Biocontrol of Halyomorpha halys. Biol. Control 2022, 176, 105068. [Google Scholar] [CrossRef]
- Talamas, E.J.; Herlihy, M.V.; Dieckhoff, C.; Hoelmer, K.A.; Buffington, M.L.; Bon, M.C.; Weber, D.C. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) Emerges in North America. J. Hymenopt. Res. 2015, 43, 119–128. [Google Scholar] [CrossRef]
- Rot, M.; Maistrello, L.; Costi, E.; Bernardinelli, I.; Malossini, G.; Benvenuto, L.; Trdan, S. Native and Non-Native Egg Parasitoids Associated with Brown Marmorated Stink Bug (Halyomorpha halys [Stål, 1855]; Hemiptera: Pentatomidae) in Western Slovenia. Insects 2021, 12, 505. [Google Scholar] [CrossRef]
- Yonow, T.; Kriticos, D.J.; Ota, N.; Avila, G.A.; Hoelmer, K.A.; Chen, H.; Caron, V. Modelling the Potential Geographic Distribution of Two Trissolcus Species for the Brown Marmorated Stink Bug, Halyomorpha halys. Insects 2021, 12, 491. [Google Scholar] [CrossRef]
- Tortorici, F.; Bombi, P.; Loru, L.; Mele, A.; Moraglio, S.T.; Scaccini, D.; Pozzebon, A.; Pantaleoni, R.A.; Tavella, L. Halyomorpha halys and Its Egg Parasitoids Trissolcus japonicus and T. mitsukurii: The Geographic Dimension of the Interaction. NeoBiota 2023, 85, 197–221. [Google Scholar] [CrossRef]
- Scaccini, D.; Falagiarda, M.; Tortorici, F.; Martinez-Sañudo, I.; Tirello, P.; Reyes-Domínguez, Y.; Gallmetzer, A.; Tavella, L.; Zandigiacomo, P.; Duso, C.; et al. An Insight into the Role of Trissolcus mitsukurii as Biological Control Agent of Halyomorpha halys in Northeastern Italy. Insects 2020, 11, 306. [Google Scholar] [CrossRef]
- Bout, A.; Tortorici, F.; Hamidi, R.; Warot, S.; Tavella, L.; Thomas, M. First Detection of the Adventive Egg Parasitoid of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Trissolcus mitsukurii (Ashmead) (Hymenoptera: Scelionidae) in France. Insects 2021, 12, 761. [Google Scholar] [CrossRef]
- Šeat, J. Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) a New Invasive Species in Serbia. Acta Entomol. Serbic 2015, 20, 167–171. [Google Scholar] [CrossRef]
- Musolin, D.L.; Konjević, A.; Karpun, N.N.; Protsenko, V.Y.; Ayba, L.Y.; Saulich, A.K. Invasive Brown Marmorated Stink Bug Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Serbia: History of Invasion, Range Expansion, Early Stages of Establishment, and First Records of Damage to Local Crops. Arthropod-Plant Interact. 2018, 12, 517–529. [Google Scholar] [CrossRef]
- Konjević, A. True Bugs (Heteroptera) as Pests in Ornamentals. In Sustainable Practices in Horticulture and Landscape Architecture; Ostojić, J., Cig, A., Eds.; Iksad: Ankara, Turkey, 2022; pp. 123–144. [Google Scholar]
- Haye, T.; Wyniger, D. Stink Bug Eggs—The Marbled Stink Bug, Halyomorpha halys. Available online: https://www.halyomorphahalys.com/wanzeneier-stink-bug-eggs.html (accessed on 7 February 2023).
- Talamas, E.J.; Buffington, M.L.; Hoelmer, K.A. Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2017, 56, 3–185. [Google Scholar] [CrossRef]
- Tortorici, F.; Talamas, E.J.; Moraglio, S.T.; Pansa, M.G.; Asadi-Farfar, M.; Tavella, L.; Caleca, V. A Morphological, Biological and Molecular Approach Reveals Four Cryptic Species of Trissolcus Ashmead (Hymenoptera, Scelionidae), Egg Parasitoids of Pentatomidae (Hemiptera). J. Hymenopt. Res. 2019, 93, 153–200. [Google Scholar] [CrossRef]
- Peng, L.; Gibson, G.A.P.; Tang, L.; Xiang, J. Review of the Species of Anastatus (Hymenoptera: Eupelmidae) Known from China, with Description of Two New Species with Brachypterous Females. Zootaxa 2020, 4767, 351–401. [Google Scholar] [CrossRef]
- Kozlov, M.A.; Kononova, S.V. Telenominae of the Fauna of the USSR; Nauka: Leningrad, Russia, 1983. [Google Scholar]
- Johnson, N.F. Systematics of Nearctic Telenomus: Classification and Revisions of the podisi and phymatae Species Groups (Hymenoptera: Scelionidae). Bull. Ohio Biol. Surv. 1984, 6, 1–113. [Google Scholar] [CrossRef]
- Sabbatini Peverieri, G.; Talamas, E.J.; Bon, M.C.; Marianelli, L.; Bernardinelli, I.; Malossini, G.; Benvenuto, L.; Roversi, P.F.; Hoelmer, K.A. Two Asian Egg Parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) Emerge in Northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2018, 67, 37–53. [Google Scholar] [CrossRef]
- Haye, T.; Wyniger, D.; Gariepy, T. Recent Range Expansion of Brown Marmorated Stink Bug in Europe. In Proceedings of the 8th International Conference on Urban Pests, Zurich, Switzerland, 20–23 July 2014; Müller, G., Pospischil, R., Robinson, W.H., Eds.; Executive Committee of the International Conference on Urban Pests: Zurich, Switzerland, 2014; pp. 309–314. [Google Scholar]
- Cesari, M.; Maistrello, L.; Ganzerli, F.; Dioli, P.; Rebecchi, L.; Guidetti, R. A Pest Alien Invasion in Progress: Potential Pathways of Origin of the Brown Marmorated Stink Bug Halyomorpha halys Populations in Italy. J. Pest Sci. 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Konjević, A. First Records of the Brown Marmorated Stink Bug Halyomorpha halys (Stål, 1855) (Hemiptera: Pentatomidae) in Republic of North Macedonia. Acta Zool. Bulg. 2020, 72, 687–690. [Google Scholar]
- Buffington, M.L.; Talamas, E.J.; Hoelmer, K.A. Team Trissolcus: Integrating Taxonomy and Biological Control to Combat the Brown Marmorated Stink Bug. Am. Entomol. 2018, 64, 224–232. [Google Scholar] [CrossRef]
- Dieckhoff, C.; Wenz, S.; Renninger, M.; Reißig, A.; Rauleder, H.; Zebitz, C.P.W.; Reetz, J.; Zimmermann, O. Add Germany to the List—Adventive Population of Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) Emerges in Germany. Insects 2021, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Noyes, J. Universal Chalcidoidea Database—World Wide Web Electronic Publication. Available online: https://www.nhm.ac.uk/our-science/data/chalcidoids/database/ (accessed on 27 March 2023).
- Konopka, J.K.; Haye, T.; Gariepy, T.D.; McNeil, J.N. Possible Coexistence of Native and Exotic Parasitoids and Their Impact on Control of Halyomorpha halys. J. Pest Sci. 2017, 90, 1119–1125. [Google Scholar] [CrossRef]
- Stahl, J.M.; Babendreier, D.; Haye, T. Life History of Anastatus bifasciatus, a Potential Biological Control Agent of the Brown Marmorated Stink Bug in Europe. Biol. Control 2019, 129, 178–186. [Google Scholar] [CrossRef]
- Mi, Q.; Zhang, J.P.; Haye, T.; Zhang, B.; Zhao, C.; Lei, Y.; Li, D.; Zhang, F. Fitness and Interspecific Competition of Trissolcus japonicus and Anastatus japonicus, Egg Parasitoids of Halyomorpha halys. Biol. Control 2021, 152, 104461. [Google Scholar] [CrossRef]
- Olson, D.M.; Wäckers, F.L. Management of Field Margins to Maximize Multiple Ecological Services. J. Appl. Ecol. 2007, 44, 13–21. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.; Pinto, D.M. Nectar-Providing Plants Enhance the Energetic State of Herbivores as Well as Their Parasitoids under Field Conditions. Ecol. Entomol. 2009, 34, 221–227. [Google Scholar] [CrossRef]
- Abram, P.K.; Mills, N.J.; Beers, E.H. Review: Classical Biological Control of Invasive Stink Bugs with Egg Parasitoids—What Does Success Look Like? Pest Manag. Sci. 2020, 76, 1980–1992. [Google Scholar] [CrossRef]
- Scala, M.; Fouani, J.M.; Zapponi, L.; Mazzoni, V.; Wells, K.E.; Biondi, A.; Baser, N.; Verrastro, V.; Anfora, G. Attraction of Egg Parasitoids Trissolcus mitsukurii and Trissolcus japonicus to the Chemical Cues of Halyomorpha halys and Nezara viridula. Insects 2022, 13, 439. [Google Scholar] [CrossRef]


| ID | Site | Habitat | Coordinates | Altitude (m asl) | Total Number of Egg Masses | Parasitized Egg Masses |
|---|---|---|---|---|---|---|
| 1 | Backi Petrovac | Hazelnut orchard | 45°19′38″ N 19°41′24″ E | 80 | 1 | Hh (1) |
| 2 | Backi Vinogradi | Semi-urban rural area, border with Hungary | 46°7′26″ N 19°51′34″ E | 95 | 2 | Hh (2) |
| 3 | Horgos | Semi-urban rural area, border with Hungary | 46°8′50″ N 19°58′11″ E | 82 | 5 | Hh (1) |
| 4 | Karavukovo | Rural area, border with Croatia | 45°30′10″ N 19°12′22″ E | 82 | 2 | Hh (1 *) |
| 5 | Lacarak | Semi-urban rural area | 44°59′49″ N 19°34′37″ E | 81 | 3 | Nv (2) |
| 6 | Ljukovo | Hazelnut orchard | 45°0′32″ N 20°0′52″ E | 101 | 306 | Hh (1) |
| 7 | Mala Remeta | Agricultural area, orchards | 45°5′40″ N 19°45′1″ E | 205 | 10 | Hh (1) |
| 8 | Novi Sad | Urban park | 45°14′23″ N 19°50′36″ E | 100 | 71 | Hh (6 *), Nv (3 *), As (1) |
| 9 | Palic | Semi-urban rural area | 46°6′14″ N 19°47′14″ E | 98 | 1 | Hh (1) |
| 10 | Ribari | Hazelnut orchard | 44°42′9″ N 19°25′10″ E | 93 | 150 | Hh (3) |
| 11 | Sombor | Walnut orchard | 45°45′53″ N 19°4′48″ E | 84 | 12 | Hh (2 *), Nv (3 *) |
| 12 | Srpski Miletic | Semi-urban rural area | 45°33′27″ N 19°12′46″ E | 83 | 5 | Nv (1) |
| Species | No. Egg Masses | No. and Species of Parasitoids Emerged in Laboratory |
|---|---|---|
| Halyomorpha halys | 19 | 49 Tba, 32 Ab, 31 Tj, 19 Tt, 10 Oo, 3 Tc, 2 Tm |
| Nezara viridula | 9 | 62 Ab, 37 Tba, 26 Ts, 1 Oo |
| Asopinae | 1 | 4 Tbe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konjević, A.; Tavella, L.; Tortorici, F. The First Records of Trissolcus japonicus (Ashmead) and Trissolcus mitsukurii (Ashmead) (Hymenoptera, Scelionidae), Alien Egg Parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) in Serbia. Biology 2024, 13, 316. https://doi.org/10.3390/biology13050316
Konjević A, Tavella L, Tortorici F. The First Records of Trissolcus japonicus (Ashmead) and Trissolcus mitsukurii (Ashmead) (Hymenoptera, Scelionidae), Alien Egg Parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) in Serbia. Biology. 2024; 13(5):316. https://doi.org/10.3390/biology13050316
Chicago/Turabian StyleKonjević, Aleksandra, Luciana Tavella, and Francesco Tortorici. 2024. "The First Records of Trissolcus japonicus (Ashmead) and Trissolcus mitsukurii (Ashmead) (Hymenoptera, Scelionidae), Alien Egg Parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) in Serbia" Biology 13, no. 5: 316. https://doi.org/10.3390/biology13050316