Protective Effects of Cannabidiol (CBD) against Qxidative Stress, but Not Excitotoxic-Related Neuronal Cell Damage—An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Primary Neuronal Cell Cultures
2.3. SH-SY5Y Cell Culture
2.4. Cell Treatment
2.5. MTT Reduction Cell Viability Assay
2.6. WST-1 Cell Viability Assay
2.7. LDH Release Assay
2.8. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.9. Measurement of Mitochondrial Membrane Potential (MMP)
2.10. Caspase-3 Activity Assay
2.11. Immunofluorescence and Hoechst 33342 Staining
2.12. Statistical Analysis
3. Results
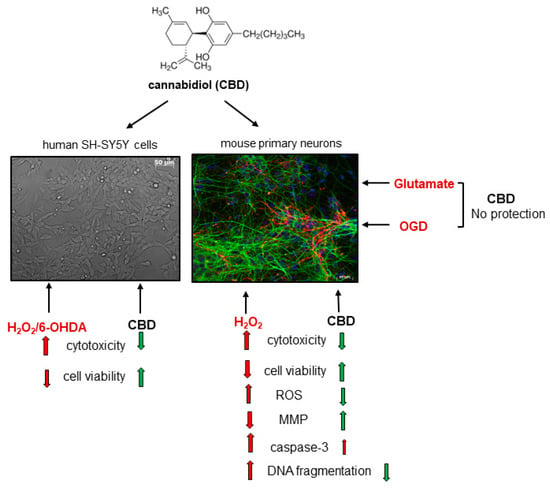
3.1. The Neuroprotective Effects of CBD in Primary Neuronal Cell Cultures
3.2. Verification of Potential Mechanisms Involved in the CBD-Mediated Neuroprotection
3.3. The Neuroprotective Effects of CBD in SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; Del Bel, E.A.; Guimarães, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef]
- Singh, K.; Bhushan, B.; Chanchal, D.K.; Sharma, S.K.; Rani, K.; Yadav, M.K.; Porwal, P.; Kumar, S.; Sharma, A.; Virmani, T.; et al. Emerging Therapeutic Potential of Cannabidiol (CBD) in Neurological Disorders: A Comprehensive Review. Behav. Neurol. 2023, 2023, 8825358. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.X.; Wang, X.; Li, B.; Xu, L.L.; Di, B. The NLRP3 inflammasome: A vital player in inflammation and mediating the anti-inflammatory effect of CBD. Inflamm. Res. 2024, 73, 227–242. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic. Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Silvestro, S.; Schepici, G.; Bramanti, P.; Mazzon, E. Molecular Targets of Cannabidiol in Experimental Models of Neurological Disease. Molecules 2020, 25, 5186. [Google Scholar] [CrossRef]
- Yousaf, M.; Chang, D.; Liu, Y.; Liu, T.; Zhou, X. Neuroprotection of Cannabidiol, Its Synthetic Derivatives and Combination Preparations against Microglia-Mediated Neuroinflammation in Neurological Disorders. Molecules 2022, 27, 4961. [Google Scholar] [CrossRef]
- Rocha, L.; Frías-Soria, C.L.; Ortiz, J.G.; Auzmendi, J.; Lazarowski, A. Is cannabidiol a drug acting on unconventional targets to control drug-resistant epilepsy? Epilepsia Open 2020, 5, 36–49. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Lolic, M.; Wink, D.; Rosenthal, R.; Axelrod, J. Neuroprotective antioxidants from marijuana. Ann. N. Y. Acad. Sci. 2000, 899, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, H.; Kang, E.K.; Ji, G.Y.; Kim, Y.; Choi, I.S. In Vitro Studies on Therapeutic Effects of Cannabidiol in Neural Cells: Neurons, Glia, and Neural Stem Cells. Molecules 2021, 26, 6077. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, C.; Prunell, G.; Narbondo, C.; de Medina, V.S.; Nadal, X.; Reyes-Parada, M.; Scorza, C. A Comparative In Vitro Study of the Neuroprotective Effect Induced by Cannabidiol, Cannabigerol, and Their Respective Acid Forms: Relevance of the 5-HT1A Receptors. Neurotox. Res. 2021, 39, 335–348. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Oberpichler-Schwenk, H.; Krieglstein, J. Primary cultures of neurons for testing neuroprotective drug effects. J. Neural Transm. Suppl. 1994, 44, 1–20. [Google Scholar] [CrossRef]
- Ioghen, O.C.; Ceafalan, L.C.; Popescu, B.O. SH-SY5Y Cell Line In Vitro Models for Parkinson Disease Research-Old Practice for New Trends. J. Integr. Neurosci. 2023, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, N.; Catanzaro, G.; Marzano, V.; Amadio, D.; Barcaroli, D.; Oddi, S.; Federici, G.; Urbani, A.; Finazzi Agrò, A.; Maccarrone, M. Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J. Biol. Chem. 2009, 284, 29413–29426. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, J.; Yao, Z.; Liu, J. Cannabidiol Induces Autophagy to Protects Neural Cells From Mitochondrial Dysfunction by Upregulating SIRT1 to Inhibits NF-κB and NOTCH Pathways. Front. Cell Neurosci. 2021, 15, 654340. [Google Scholar] [CrossRef]
- Yordanov, Y.; Stefanova, D.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Konstantinov, S.; Yoncheva, K. Formulation of Nanomicelles Loaded with Cannabidiol as a Platform for Neuroprotective Therapy. Pharmaceutics 2022, 14, 2625. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lasoń, W. Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition. Biomolecules 2020, 10, 1530. [Google Scholar] [CrossRef]
- Domin, H.; Jantas, D.; Śmiałowska, M. Neuroprotective effects of the allosteric agonist of metabotropic glutamate receptor 7 AMN082 on oxygen-glucose deprivation- and kainate-induced neuronal cell death. Neurochem. Int. 2015, 88, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Warszyński, P.; Lasoń, W. Carnosic Acid Shows Higher Neuroprotective Efficiency than Edaravone or Ebselen in In Vitro Models of Neuronal Cell Damage. Molecules 2024, 29, 119. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Piotrowski, M.; Lason, W. An Involvement of PI3-K/Akt Activation and Inhibition of AIF Translocation in Neuroprotective Effects of Undecylenic Acid (UDA) against Pro-Apoptotic Factors-Induced Cell Death in Human Neuroblastoma SH-SY5Y Cells. J. Cell Biochem. 2015, 116, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.Y.; Seo, J.; Choi, I.S. Neuroprotective Effect of Cannabidiol against Hydrogen Peroxide in Hippocampal Neuron Culture. Cannabis Cannabinoid Res. 2021, 6, 40–47. [Google Scholar] [CrossRef]
- Romariz, S.A.A.; Sanabria, V.; da Silva, K.R.; Quintella, M.L.; de Melo, B.A.G.; Porcionatto, M.; de Almeida, D.C.; Longo, B.M. High Concentrations of Cannabidiol Induce Neurotoxicity in Neurosphere Culture System. Neurotox. Res. 2024, 42, 14. [Google Scholar] [CrossRef] [PubMed]
- Schönhofen, P.; de Medeiros, L.M.; Bristot, I.J.; Lopes, F.M.; De Bastiani, M.A.; Kapczinski, F.; Crippa, J.A.; Castro, M.A.; Parsons, R.B.; Klamt, F. Cannabidiol Exposure during Neuronal Differentiation Sensitizes Cells against Redox-Active Neurotoxins. Mol. Neurobiol. 2015, 52, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.T.; Lairion, F.; Repetto, M.; Ettcheto, M.; Merelli, A.; Lazarowski, A.; Auzmendi, J. Cannabidiol (CBD) Alters the Functionality of Neutrophils (PMN). Implications in the Refractory Epilepsy Treatment. Pharmaceuticals 2021, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087361. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Candé, C.; Cecconi, F.; Dessen, P.; Kroemer, G. Apoptosis-inducing factor (AIF): Key to the conserved caspase-independent pathways of cell death? J. Cell Sci. 2002, 115, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002, 297, 259–263. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Lau, W.K.; Yu, M.S.; Lai, C.S.; Yeung, S.C.; So, K.F.; Chang, R.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Mendivil-Perez, M.; Felizardo-Otalvaro, A.A.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Cannabidiol Protects Dopaminergic-like Neurons against Paraquat- and Maneb-Induced Cell Death through Safeguarding DJ-1CYS106 and Caspase 3 Independently of Cannabinoid Receptors: Relevance in Parkinson’s Disease. ACS Chem. Neurosci. 2023, 14, 2159–2171. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Pollastro, F.; Bramanti, P.; Mazzon, E. Cannabidiol exerts protective effects in an in vitro model of Parkinson’s disease activating AKT/mTOR pathway. Fitoterapia 2020, 143, 104553. [Google Scholar] [CrossRef]
- Lyons, E.L.; Leone-Kabler, S.; Kovach, A.L.; Thomas, B.F.; Howlett, A.C. Cannabinoid receptor subtype influence on neuritogenesis in human SH-SY5Y cells. Mol. Cell Neurosci. 2020, 109, 103566. [Google Scholar] [CrossRef]
- Marsicano, G.; Moosmann, B.; Hermann, H.; Lutz, B.; Behl, C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J. Neurochem. 2002, 80, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Landucci, E.; Mazzantini, C.; Lana, D.; Davolio, P.L.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids. Int. J. Mol. Sci. 2021, 22, 9773. [Google Scholar] [CrossRef] [PubMed]
- Lana, D.; Landucci, E.; Mazzantini, C.; Magni, G.; Pellegrini-Giampietro, D.E.; Giovannini, M.G. The Protective Effect of CBD in a Model of In Vitro Ischemia May Be Mediated by Agonism on TRPV2 Channel and Microglia Activation. Int. J. Mol. Sci. 2022, 23, 12144. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017, 11, 577–585. [Google Scholar] [CrossRef]
- Maguire, R.F.; Wilkinson, D.J.; England, T.J.; O’Sullivan, S.E. The Pharmacological Effects of Plant-Derived versus Synthetic Cannabidiol in Human Cell Lines. Med. Cannabis Cannabinoids 2021, 4, 86–96. [Google Scholar] [CrossRef]
- Borgonetti, V.; Biagi, M.; Galeotti, N.; Manetti, F.; Governa, P. Investigation on the neuroprotective effect of a cannabidiol-enriched non-psychotropic Cannabis sativa L. extract in an in vitro model of excitotoxicity. Fitoterapia 2022, 163, 105315. [Google Scholar] [CrossRef]
- Assadpour, E.; Rezaei, A.; Das, S.S.; Krishna Rao, B.V.; Singh, S.K.; Kharazmi, M.S.; Jha, N.K.; Jha, S.K.; Prieto, M.A.; Jafari, S.M. Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications. Pharmaceuticals 2023, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, M.; Simko, P.; Urbanska, N.; Kiskova, T. Exploring the Potential of Cannabinoid Nanodelivery Systems for CNS Disorders. Pharmaceutics 2023, 15, 204. [Google Scholar] [CrossRef]
- Cunha, J.M.; Carlini, E.A.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 1980, 21, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.; Zuardi, A.W.; Crippa, J.A. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantas, D.; Leśkiewicz, M.; Regulska, M.; Procner, M.; Warszyński, P.; Lasoń, W. Protective Effects of Cannabidiol (CBD) against Qxidative Stress, but Not Excitotoxic-Related Neuronal Cell Damage—An In Vitro Study. Biomolecules 2024, 14, 564. https://doi.org/10.3390/biom14050564
Jantas D, Leśkiewicz M, Regulska M, Procner M, Warszyński P, Lasoń W. Protective Effects of Cannabidiol (CBD) against Qxidative Stress, but Not Excitotoxic-Related Neuronal Cell Damage—An In Vitro Study. Biomolecules. 2024; 14(5):564. https://doi.org/10.3390/biom14050564
Chicago/Turabian StyleJantas, Danuta, Monika Leśkiewicz, Magdalena Regulska, Magdalena Procner, Piotr Warszyński, and Władysław Lasoń. 2024. "Protective Effects of Cannabidiol (CBD) against Qxidative Stress, but Not Excitotoxic-Related Neuronal Cell Damage—An In Vitro Study" Biomolecules 14, no. 5: 564. https://doi.org/10.3390/biom14050564