Microbiological Mechanisms of Collaborative Remediation of Cadmium-Contaminated Soil with Bacillus cereus and Lawn Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Impact of Bacillus cereus Application Rate on Cd-Contaminated Soil
2.1.1. The Effects of Bacillus cereus Application Rate on Soil Physicochemical Properties
2.1.2. The Effect of Bacillus cereus Application Rate on Soil Cd Content
2.1.3. The Effect of Bacillus cereus Application Rate on the Forms of Cd in Rhizosphere Soil
2.2. The Effect of Bacillus cereus Application Rate on Plant Growth and Cd Absorption
2.3. The Effect of Bacillus cereus Application Rate on the Microbial Structure of Heavy-Metal-Contaminated Soil
2.3.1. The Variation in Bacterial Community Alpha Diversity between Rhizosphere and Non-Rhizosphere Soil
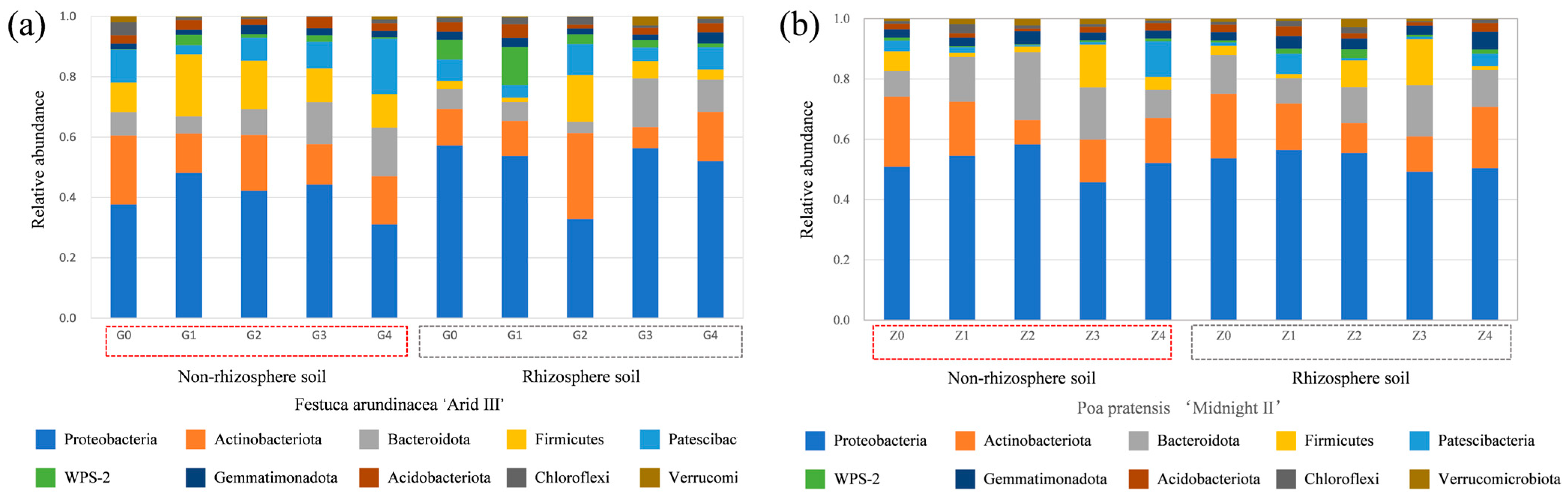
2.3.2. The Changes in the Relative Abundance of Bacterial Species
2.3.3. Biomarker Identification and LEfSe Analysis
2.4. The Response Relationship between Plant Remediation and Soil Microbial Ecological Characteristics
3. Materials and Methods
3.1. Experimental Materials
3.2. Experimental Design and Sample Collection
3.3. Measurement Methods
3.3.1. Soil Physicochemical Properties
3.3.2. Plant Biomass and Heavy Metal Accumulation
3.3.3. Soil Microbial Structure
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Wang, D.; Tao, Y.; Shen, M.; Wei, W.; Cai, C.; Ding, C.; Li, J.; Song, L.; Yin, B.; et al. Effects of elevated CO2 on the Cd uptake by rice in Cd-contaminated paddy soils. J. Hazard. Mater. 2023, 442, 130140. [Google Scholar] [CrossRef]
- Liu, S.; Ali, S.; Yang, R.; Tao, J.; Ren, B. A newly discovered Cd-hyperaccumulator Lantana camara L. J. Hazard. Mater. 2019, 371, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, S.; Guo, Y.; Zhu, Y.; Agathokleous, E.; Ahmad, S.; Han, J. Cd induced biphasic response in soil alkaline phosphatase and changed soil bacterial community composition: The role of background Cd contamination and time as additional factors. Sci. Total. Environ. 2020, 757, 143771. [Google Scholar] [CrossRef] [PubMed]
- Khatereh, N.; Ali, A.; Behnam Asgari, L.; van Hullebusch, E.D. Effects of biochar dose on cadmium accumulation in spinach and its fractionation in a calcareous soil. Arab. J. Geosci. 2022, 15, 336. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.W.; Zia-Ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.G.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Grobelak, A.; Napora, A. The Chemophytostabilisation Process of Heavy Metal Polluted Soil. PLoS ONE 2015, 10, e0129538. [Google Scholar] [CrossRef]
- Xiao, L.; Bi, Y.; Du, S.; Wang, Y.; Guo, C.; Christie, P. Response of ecological stoichiometry and stoichiometric homeostasis in the plant-litter-soil system to re-vegetation type in arid mining subsidence areas. J. Arid. Environ. 2020, 184, 104298. [Google Scholar] [CrossRef]
- Nayeri, S.; Dehghanian, Z.; Asgari Lajayer, B.; Thomsson, A.; Astatkie, T.; Price, G.W. CRISPR/Cas9-Mediated genetically edited ornamental and aromatic plants: A promising technology in phytoremediation of heavy metals. J. Clean. Prod. 2023, 428, 139512. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, Z.; Liu, C.; Liu, J.; Zhuang, J. Enhancing remediation potential of heavy metal contaminated soils through synergistic application of microbial inoculants and legumes. Front. Microbiol. 2023, 14, 1272591. [Google Scholar] [CrossRef]
- Gerami, Z.; Lakzian, A.; Hemati, A.; Amirifar, A.; Asgari Lajayer, B.; van Hullebusch, E.D. Effect of cadmium on sorghum root colonization by glomeral fungi and its impact on total and easily extractable glomalin production. Environ. Sci. Pollut. Res. 2021, 28, 34570–34583. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Q.; Liu, W.; Zhong, D.; Ge, Y.; Christie, P.; Luo, Y. Soil microbial community and association network shift induced by several tall fescue cultivars during the phytoremediation of a petroleum hydrocarbon-contaminated soil. Sci. Total. Environ. 2021, 792, 148411. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Ae, N.; Freitas, H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Srivastava, N.; Pal, D.B.; Alkhanani, M.F.; Almalki, A.H.; Areeshi, M.Y.; Naidu, R.; Gupta, V.K. Functional microbiome strategies for the bioremediation of petroleum-hydrocarbon and heavy metal contaminated soils: A review. Sci. Total. Environ. 2022, 833, 155222. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Kailu, Z.; Yang, Z.; Dong, L.; Xiaoyan, S.; Li, L.; Huiping, F.; Qing, H.; Zhiqiang, Z. Screening of cadmium-chromium-tolerant strains and synergistic remediation of heavy metal-contaminated soil using king grass combined with highly efficient microbial strains. Sci. Total. Environ. 2023, 912, 168990. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Yu, M.; Xu, H.; Huang, S.; Wang, Z.; Zhang, X.-X. Characterization of cadmium biosorption by inactive biomass of two cadmium-tolerant endophytic bacteria Microbacterium sp. D2-2 and Bacillus sp. C9-3. Ecotoxicology 2021, 30, 1419–1428. [Google Scholar] [CrossRef]
- Wang, X.; Cai, D.; Ji, M.; Chen, Z.; Yao, L.; Han, H. Isolation of heavy metal-immobilizing and plant growth-promoting bacteria and their potential in reducing Cd and Pb uptake in water spinach. Sci. Total. Environ. 2022, 819, 153242. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Lin, H.-L. Remediation of soil contaminated with the heavy metal (Cd2+). J. Hazard. Mater. 2005, 122, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bu, H.; Feng, Q.; Wassie, M.; Amee, M.; Jiang, Y.; Bi, Y.; Hu, L.; Chen, L. Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass. Environ. Res. 2021, 200, 111730. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2019, 254, 109779. [Google Scholar] [CrossRef]
- Lanping, S.; Xiaoyang, X.; Yuanyuan, Z.; Wanyue, H.; Xiaoping, L.; Yanmei, A.; Yang, W.; Zekun, Z.; Hong, C.; Yongjie, H.; et al. Dynamic mechanisms of cadmium accumulation and detoxification by Lolium perenne grown in soil inoculated with the cadmium-tolerant bacterium strain Cdq4-2. Sci. Total. Environ. 2023, 873, 162314. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: Current knowledge and future directions. Sci. Total. Environ. 2022, 838, 156435. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.; Xie, S.; Awadelkareem, W.; Wang, J.; Qian, X. Influence of biofertilizer on heavy metal bioremediation and enzyme activities in the soil to revealing the potential for sustainable soil restoration. Sci. Rep. 2023, 13, 20684. [Google Scholar] [CrossRef]
- Chen, B.; Tan, S.; Zeng, Q.; Wang, A.; Zheng, H. Soil nutrient heterogeneity affects the accumulation and transfer of cadmium in Bermuda grass (Cynodon dactylon (L.) pers.). Chemosphere 2019, 221, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, B.; Dong, Y.; Lin, H. Endophyte colonization enhanced cadmium phytoremediation by improving endosphere and rhizosphere microecology characteristics. J. Hazard. Mater. 2022, 434, 128829. [Google Scholar] [CrossRef]
- Li, K.-T.; Yang, Y.; Zhang, S.-W.; Cheng, X. Dynamics of the Bacterial Community’s Soil during the In-Situ Degradation Process of Waste Chicken Feathers. Indian J. Microbiol. 2022, 62, 225–233. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Sun, Q.; Zhang, M.; Ahmad, I. Inoculation of Bacillus spp. Modulate the Soil Bacterial Communities and Available Nutrients in the Rhizosphere of Vetiver Plant Irrigated with Acid Mine Drainage. Chemosphere 2020, 263, 128345. [Google Scholar] [CrossRef]
- Li, B.; Wu, B.; Dong, Y.; Lin, H.; Liu, C. Endophyte inoculation enhanced microbial metabolic function in the rhizosphere benefiting cadmium phytoremediation by Phytolacca acinosa. Chemosphere 2023, 338, 139421. [Google Scholar] [CrossRef] [PubMed]
- Farh, M.E.-A.; Kim, Y.-J.; Sukweenadhi, J.; Singh, P.; Yang, D.-C. Aluminium resistant, plant growth promoting bacteria induce overexpression of Aluminium stress related genes in Arabidopsis thaliana and increase the ginseng tolerance against Aluminium stress. Microbiol. Res. 2017, 200, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Ma, J.; Liu, Q.; Shi, T.; Gong, Y.; Yang, S.; Wu, Y. Status of chromium accumulation in agricultural soils across China (1989–2016). Chemosphere 2020, 256, 127036. [Google Scholar] [CrossRef]
- Li, Q.; Xing, Y.; Fu, X.; Ji, L.; Li, T.; Wang, J.; Chen, G.; Qi, Z.; Zhang, Q. Biochemical mechanisms of rhizospheric Bacillus subtilis-facilitated phytoextraction by alfalfa under cadmium stress—Microbial diversity and metabolomics analyses. Ecotoxicol. Environ. Saf. 2021, 212, 112016. [Google Scholar] [CrossRef]
- Jeong, S.; Moon, H.S.; Shin, D.; Nam, K. Survival of introduced phosphate-solubilizing bacteria (PSB) and their impact on microbial community structure during the phytoextraction of Cd-contaminated soil. J. Hazard. Mater. 2013, 263, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.L.; Tang, C.; Franks, A.E. Microbial associated plant growth and heavy metal accumulation to improve phytoextraction of contaminated soils. Soil Biol. Biochem. 2016, 103, 131–137. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Nai, F.; Rajkumar, M.; Luo, Y.; Rocha, I.; Freitas, H. The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. J. Environ. Manag. 2015, 156, 62–69. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly isolated Bacillus sp. PS-6 assisted phytoremediation of heavy metals using Phragmites communis: Potential application in wastewater treatment. Bioresour. Technol. 2020, 320, 124353. [Google Scholar] [CrossRef]
- Huang, G.; Zhou, X.; Guo, G.; Ren, C.; Rizwan, M.S.; Islam, M.S.; Hu, H. Variations of dissolved organic matter and Cu fractions in rhizosphere soil induced by the root activities of castor bean. Chemosphere 2020, 254, 126800. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.-y.; Wang, D. Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus annuus L.) enhanced with biodegradable chelating agents. J. Clean. Prod. 2020, 263, 121491. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Effect of cadmium, copper and zinc on plants, soil microorganisms and soil enzymes. J. Elem. 2013, 18, 769–796. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Fernández, C.; Escuer, M.; Campos-Herrera, R.; Beltrán Rodríguez, M.E.; Carbonell, G.; Rodríguez Martín, J.A. Effect of soil properties, heavy metals and emerging contaminants in the soil nematodes diversity. Environ. Pollut. 2016, 213, 184–194. [Google Scholar] [CrossRef]
- Bettermann, A.; Zethof, J.H.T.; Babin, D.; Cammeraat, E.L.H.; Solé-Benet, A.; Lázaro, R.; Luna, L.; Nesme, J.; Sørensen, S.J.; Kalbitz, K.; et al. Importance of microbial communities at the root-soil interface for extracellular polymeric substances and soil aggregation in semiarid grasslands. Soil Biol. Biochem. 2021, 159, 108301. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Lima, A.I.G.; Figueira, E.M.d.A.P. Screening Possible Mechanisms Mediating Cadmium Resistance in Rhizobium leguminosarum bv. viciae Isolated from Contaminated Portuguese Soils. Microb. Ecol. 2006, 52, 176–186. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, L.; Liu, X.; Zhang, S.; Zhang, L.; Qiu, Z.; Yu, C. Microbial community succession in soils under long-term heavy metal stress from community diversity-structure to KEGG function pathways. Environ. Res. 2022, 214, 113822. [Google Scholar] [CrossRef] [PubMed]
- Kou, B.; He, Y.; Wang, Y.; Qu, C.; Tang, J.; Wu, Y.; Tan, W.; Yuan, Y.; Yu, T. The relationships between heavy metals and bacterial communities in a coal gangue site. Environ. Pollut. 2023, 322, 121136. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yao, J.; Chen, Z.; Ma, B.; Li, H.; Wancheng, P.; Liu, J.; Wang, D.; Duran, R. Biogeography, assembly processes and species coexistence patterns of microbial communities in metalloids-laden soils around mining and smelting sites. J. Hazard. Mater. 2021, 425, 127945. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, D.; Tang, W.; Wang, L.; Li, Q.; Lu, Z.; Liu, H.; Zhong, Y.; He, T.; Guo, S. Phytoremediation of cadmium-polluted soil assisted by D-gluconate-enhanced Enterobacter cloacae colonization in the Solanum nigrum L. rhizosphere. Sci. Total. Environ. 2020, 732, 139265. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M. Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: A review. 3 Biotech 2014, 5, 111–121. [Google Scholar] [CrossRef]
- Manickam, N.; Bajaj, A.; Saini, H.S.; Shanker, R. Surfactant mediated enhanced biodegradation of hexachlorocyclohexane (HCH) isomers by Sphingomonas sp. NM05. Biodegradation 2012, 23, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, X.; Ling, Q.; Li, S.; Wei, J.; Xin, M.; Xie, D.; Chen, X.; Liu, K.; Yu, F. Bacterial extracellular polymeric substances: Impact on soil microbial community composition and their potential role in heavy metal-contaminated soil. Ecotoxicol. Environ. Saf. 2022, 240, 113701. [Google Scholar] [CrossRef]
- Han, H.; Wu, X.; Yao, L.; Chen, Z. Heavy metal-immobilizing bacteria combined with calcium polypeptides reduced the uptake of Cd in wheat and shifted the rhizosphere bacterial communities. Environ. Pollut. 2020, 267, 115432. [Google Scholar] [CrossRef]
- Kamal, N.; Liu, Z.; Qian, C.; Wu, J.; Zhong, X. Improving hybrid Pennisetum growth and cadmium phytoremediation potential by using Bacillus megaterium BM18-2 spores as biofertilizer. Microbiol. Res. 2020, 242, 126594. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Huang, J.; Wang, H.; Tang, D. Effects of soil heavy metal pollution on microbial activities and community diversity in different land use types in mining areas. Environ. Sci. Pollut. Res. 2020, 27, 20215–20226. [Google Scholar] [CrossRef]
- Jia, R. Microbially-Enhanced Phytoremediation of Acidiccadmium-Contaminated Soil by Turfgrass and It Smechanism; Xi’an University of Technology: Xi’an, China, 2023. [Google Scholar]
- Jalali, M.; Jalali, M. Sewage Sludge Application Effects on Phosphorus Uptake by Cucumber and on Rhizosphere and Non-rhizosphere Soils Under Greenhouse Conditions. J. Soil Sci. Plant Nutr. 2021, 21, 596–611. [Google Scholar] [CrossRef]
- Wang, L.; Lin, H.; Dong, Y.; Li, B.; He, Y. Effects of endophytes inoculation on rhizosphere and endosphere microecology of Indian mustard (Brassica juncea) grown in vanadium-contaminated soil and its enhancement on phytoremediation. Chemosphere 2019, 240, 124891. [Google Scholar] [CrossRef] [PubMed]






| Lawn Plants | Handle | Concentration (CFU/mL) | Cd Concentration (mg/kg) | Translocation Factor (TF) | Bioconcentration Factor (BCF) | |
|---|---|---|---|---|---|---|
| Aboveground | Root | |||||
| Festuca arundinacea ‘Arid Ⅲ’ | G0 | 0 | 0.481 ± 0.012 d | 1.907 ± 0.061 d | 0.255 ± 0.003 d | 0.431 ± 0.006 c |
| G1 | 103 | 0.753 ± 0.013 c | 2.557 ± 0.08 b | 0.294 ± 0.006 c | 0.603 ± 0.009 b | |
| G2 | 105 | 1.256 ± 0.139 a | 2.682 ± 0.093 a | 0.467 ± 0.039 a | 0.898 ± 0.031 a | |
| G3 | 107 | 0.992 ± 0.018 b | 2.489 ± 0.039 c | 0.398 ± 0.007 b | 0.877 ± 0.010 a | |
| G4 | 109 | 0.756 ± 0.014 c | 2.557 ± 0.082 b | 0.308 ± 0.005 c | 0.673 ± 0.011 b | |
| Poa pratensis ‘Midnight Ⅱ’ | Z0 | 0 | 0.323 ± 0.027 e | 1.895 ± 0.031 d | 0.170 ± 0.012 d | 0.221 ± 0.006 d |
| Z1 | 103 | 0.651 ± 0.038 b | 2.385 ± 0.069 b | 0.273 ± 0.014 b | 0.530 ± 0.013 b | |
| Z2 | 105 | 0.851 ± 0.043 a | 2.526 ± 0.136 a | 0.339 ± 0.031 a | 0.499 ± 0.029 a | |
| Z3 | 107 | 0.529 ± 0.018 c | 2.065 ± 0.045 c | 0.256 ± 0.014 c | 0.452 ± 0.015 c | |
| Z4 | 109 | 0.469 ± 0.041 d | 1.825 ± 0.026 d | 0.257 ± 0.021 c | 0.456 ± 0.017 c | |
| Treatment | Chao1 Index | Shannon Index | Simpson Index | Coverage | ||
|---|---|---|---|---|---|---|
| Festuca arundinacea ‘Arid Ⅲ’ | Non-rhizosphere soil | G0 | 649.76 ± 2.23 i | 7.27 ± 0.03 g | 0.956 ± 0.005 f | 0.987 |
| G1 | 687.29 ± 2.38 h | 7.40 ± 0.02 f | 0.977 ± 0.005 ab | 0.990 | ||
| G2 | 845.55 ± 4.93 d | 7.61 ± 0.04 d | 0.980 ± 0.001 a | 0.998 | ||
| G3 | 729.80 ± 5.27 g | 7.45 ± 0.01 e | 0.971 ± 0.002 c | 0.986 | ||
| G4 | 752.73 ± 3.89 f | 7.59 ± 0.06 de | 0.975 ± 0.003 b | 0.993 | ||
| Rhizosphere soil | G0 | 1025.64 ± 2.76 a | 8.57 ± 0.03 a | 0.966 ± 0.001 d | 0.988 | |
| G1 | 1003.04 ± 3.86 b | 8.56 ± 0.04 a | 0.960 ± 0.001 e | 0.997 | ||
| G2 | 820.59 ± 3.58 de | 8.48 ± 0.04 b | 0.961 ± 0.002 e | 0.998 | ||
| G3 | 795.01 ± 3.72 e | 7.95 ± 0.03 c | 0.958 ± 0.003 ef | 0.988 | ||
| G4 | 973.20 ± 4.06 c | 8.52 ± 0.02 ab | 0.965 ± 0.002 d | 0.989 | ||
| Poa pratensis ‘Midnight Ⅱ’ | Non-rhizosphere soil | Z0 | 601.36 ± 2.58 h | 6.67 ± 0.04 h | 0.957 ± 0.003 g | 0.990 |
| Z1 | 646.55 ± 3.40 f | 6.75 ± 0.02 g | 0.962 ± 0.002 f | 0.998 | ||
| Z2 | 761.03 ± 4.23 e | 6.94 ± 0.01 ef | 0.988 ± 0.001 a | 0.988 | ||
| Z3 | 816.12 ± 1.917 d | 7.33 ± 0.02 c | 0.965 ± 0.001 e | 0.993 | ||
| Z4 | 873.15 ± 2.99 b | 7.91 ± 0.03 a | 0.975 ± 0.001 d | 0.994 | ||
| Rhizosphere soil | Z0 | 916.03 ± 7.91 a | 7.75 ± 0.02 b | 0.980 ± 0.003 b | 0.986 | |
| Z1 | 828.77 ± 4.80 c | 7.72 ± 0.03 b | 0.981 ± 0.005 b | 0.989 | ||
| Z2 | 643.00 ± 2.74 f | 7.03 ± 0.02 e | 0.979 ± 0.002 bc | 0.985 | ||
| Z3 | 910.36 ± 4.69 ab | 6.89 ± 0.02 f | 0.975 ± 0.002 d | 0.991 | ||
| Z4 | 634.33 ± 2.71 g | 7.14 ± 0.04 d | 0.977 ± 0.002 c | 0.992 | ||
| pH | Organic Matter (OM, g/kg) | Total Nitrogen (TN, g/kg) | Total Potassium (TP, g/kg) | Total Carbon (TC, g/kg) | Available Phosphorus (AP, mg/kg) | Total Cd (T-Cd, mg/kg) |
|---|---|---|---|---|---|---|
| 4.32 | 9.26 | 0.40 | 25.06 | 6.17 | 4.58 | 2.31 |
| Lawn Plants | Experimental Code | Inoculation Dosage | Viable Count |
|---|---|---|---|
| Poa pratensis ‘Midnight Ⅱ’ | Z0 | 20 mL | 0 CFU/mL |
| Z1 | 20 mL | 103 CFU/mL | |
| Z2 | 20 mL | 105 CFU/mL | |
| Z3 | 20 mL | 107 CFU/mL | |
| Z4 | 20 mL | 109 CFU/mL | |
| Festuca arundinacea ‘Arid Ⅲ’ | G0 | 20 mL | 0 CFU/mL |
| G1 | 20 mL | 103 CFU/mL | |
| G2 | 20 mL | 105 CFU/mL | |
| G3 | 20 mL | 107 CFU/mL | |
| G4 | 20 mL | 109 CFU/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Yang, Z.; Chen, X.; Jia, R.; Yao, S.; Gan, B.; Fan, D.; Yang, X.; Li, W.; Chen, Y. Microbiological Mechanisms of Collaborative Remediation of Cadmium-Contaminated Soil with Bacillus cereus and Lawn Plants. Plants 2024, 13, 1303. https://doi.org/10.3390/plants13101303
Zhou B, Yang Z, Chen X, Jia R, Yao S, Gan B, Fan D, Yang X, Li W, Chen Y. Microbiological Mechanisms of Collaborative Remediation of Cadmium-Contaminated Soil with Bacillus cereus and Lawn Plants. Plants. 2024; 13(10):1303. https://doi.org/10.3390/plants13101303
Chicago/Turabian StyleZhou, Beibei, Zehao Yang, Xiaopeng Chen, Ruonan Jia, Shaoxiong Yao, Bin Gan, Dongliang Fan, Xie Yang, Wenqian Li, and Yunhan Chen. 2024. "Microbiological Mechanisms of Collaborative Remediation of Cadmium-Contaminated Soil with Bacillus cereus and Lawn Plants" Plants 13, no. 10: 1303. https://doi.org/10.3390/plants13101303




