Effect of Exogenous Organic Matter on Phosphorus Forms in Middle-High Fertility Cinnamon Soil
Abstract
:1. Results and Analysis
1.1. Effects of EOM on Soil Phosphatase Activity
1.1.1. Effects of EOM on Soil Neutral Phosphatase Activity
1.1.2. Effects of EOM on Soil Alkaline Phosphatase Activity
1.1.3. Effects of EOM on Soil Acid Phosphatase Activity
1.2. Effects of EOM on Soil Available P Content
1.3. Effects of EOM on the Content of Inorganic P in Soil
1.3.1. Effects of EOM on Soil Ca2-P Content
1.3.2. Effect of EOM on Ca8-P Content
1.3.3. Effect of EOM on Soil Al-P Content
1.3.4. Effect of EOM on Soil Fe-P Content
1.3.5. Effect of Different EOMs on Soil O-P Content
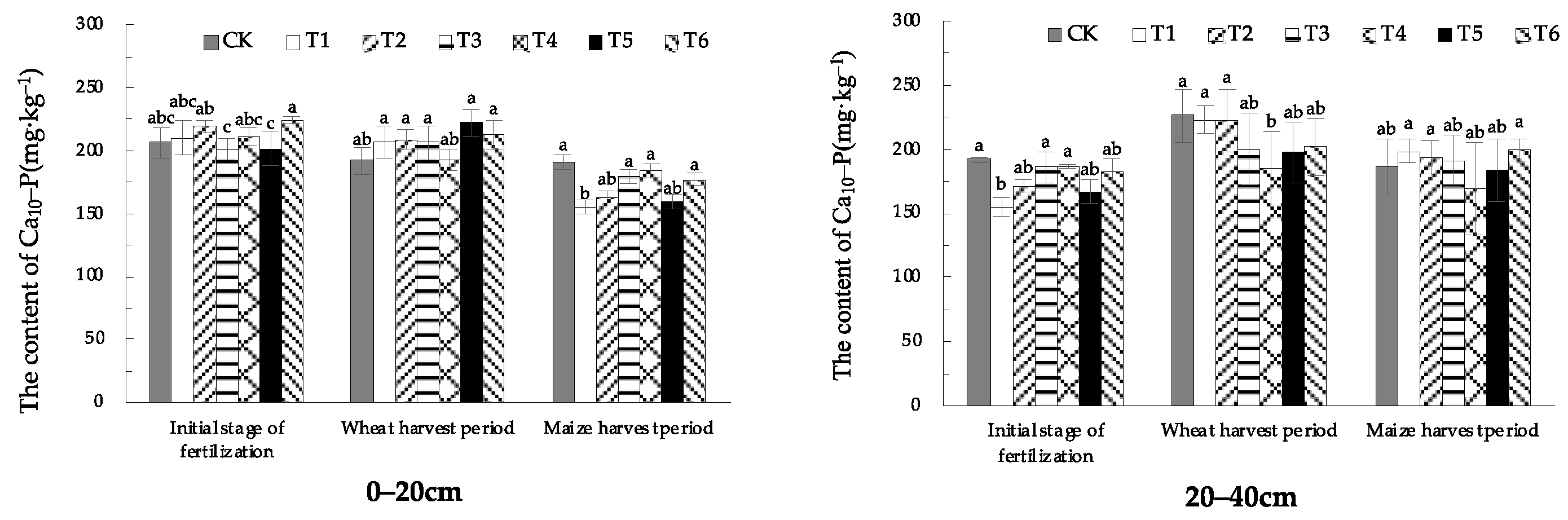
1.3.6. Effect of EOM on Soil Ca10-P Content
1.4. Correlation Analysis of Different Phosphorus Forms with Phosphatase Activity
2. Materials and Methods
2.1. General Description of the Experiment Site
2.2. Test Materials
2.3. Sample Collection and Determination
2.4. Data Processing
3. Discussion
3.1. Effect of EOM on Phosphorus Speciation
3.2. Effect of EOM on Soil Phosphatase Activity
3.3. Effect of EOM on the Blockage of Phosphorus Leaching
4. Conclusions
- (1)
- EOM promoted the conversion of phosphorus to moderately soluble phosphorus (Ca8-P, Al-P, Fe-P), and slowed down the conversion of phosphorus to closed-accumulation phosphorus O-P and mineralized phosphorus Ca10-P to a certain extent. 4000 kg·hm−2 of cow manure-vermicompost treatment was the best for the promotion of the conversion of phosphorus to Ca2-P in the surface layer of the soil.
- (2)
- EOM played a positive role in increasing the activity of soil neutral and acid phosphatase, promoting the conversion of organic phosphorus to inorganic phosphorus, and increasing the content of effective phosphorus, and 59.97 kg·hm−2 fulvic acid treatment was generally more effective.
- (3)
- Fulvic acid, biochar, microbial fertilizer, cow manure-vermicompost and pig manure-vermicompost were all effective in activating and retaining effective phosphorus in the soil. 900 kg·hm−2 biochar treatment was the most effective in preventing downward phosphorus loss from the surface layer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, C.X.; Liu, H.E.; Nie, Z.; Li, C.; Qin, S.Y.; Liu, H.Y.; Xu, J.Y.; Zhao, P. Effect of phosphorus combined selenium application on selenium absorption of winter wheat and transformation of selenium chemical forms of in soil. J. Nucl. Agric. Sci. 2023, 37, 379–388. [Google Scholar]
- Ye, G.K.; E Sheng, Z.; Chen, Z.Y.; Yuan, J.H.; Lu, G.B.; Zhang, P.; Liu, Y.N.; Zhao, T.X.; Wang, Y.X. The forms and classification methods of phosphorus in soil: Research progress. Chin. Agric. Sci. Bull. 2023, 39, 96–102. [Google Scholar]
- Tian, Y.; Liu, J.; Zhang, T.T.; Wang, R.Z.; Hui, X.L.; Li, C.; Ma, Q.X.; Wang, Z.H. Effects of long-term phosphorus application on winter wheat yield and soil inorganic phosphorus forms in dryland. J. Plant Nutr. Fertil. 2022, 28, 94–103. [Google Scholar]
- Xu, Z.; Wang, W.X.; Xu, L.; Yi, K.K. Research progress in molecular mechanism of rice phosphorus uptake and translocation. J. Plant Nutr. Fertil. 2018, 24, 1378–1385. [Google Scholar]
- Chang, S.C.; Jackson, M.L. Fractionation of soil phosphorus. Soil Sci. 1957, 84, 133–144. [Google Scholar] [CrossRef]
- Gu, Y.C.; Jiang, B.F. Method for determination of inorganic phosphorus fractionation in calcareous soils. Soil 1990, 2, 101–102+110. [Google Scholar]
- Yao, S.; Zhang, D.J.; Batbayar, J.; Liu, L.; Li, R.N.; Zhou, J.C.; Zhang, S.L.; Yang, X.Y. Responses of phosphorus use efficiency to soil phosphorus fertility under winter wheat-summer maize cropping in loess soil. J. Plant Nutr. Fertil. 2018, 24, 1640–1650. [Google Scholar]
- Liu, Y.X.; Yang, X.; Yang, S.M.; Lv, H.H.; Wang, Y.Y. Review on the effect of biochar on soil phosphorus transformation and mechanisms. J. Plant Nutr. Fertil. 2016, 22, 1690–1695. [Google Scholar]
- Zhang, W.W. The Difference and Mechanism of the Relation between Soil Olsen-P and P Budget of Fluvo-Aquic Soil under Long-Term Fertilization; Chinese Academy of Agricultural Sciences: Beijing, China, 2020; pp. 3–19. [Google Scholar]
- Tian, C.; Yu, Y.J.; Wu, L.L.; Zhang, L.; Huang, J.; Zhu, L.F.; Zhang, J.H.; Zhu, C.Q.; Kong, Y.L.; Wu, M.Y.; et al. Effects of various irrigation and fertilization schedules on the transformation and availability of phosphorus in paddy fields. Trans. Chin. Soc. Agric. Eng. 2021, 37, 112–122. [Google Scholar]
- Zhang, L.L.; Shi, Q.H.; Gong, B. Application of Fulvic Acid and Phosphorus Fertilizer on Tomato Growth, Development, and Phosphorus Utilization in Neutral and Alkaline Soil. Sci. Agric. Sin. 2020, 53, 3567–3575. [Google Scholar]
- Liu, X.Y.; Yang, J.S.; Yao, R.J. Synergistic effects of fertilizer reduction and fulvic acid application on decreasing NaCl content and N, P availability of salinized soil. J. Plant Nutr. Fertil. 2021, 27, 1339–1350. [Google Scholar]
- Sarfraz, R.; Yang, W.H.; Wang, S.S.; Zhou, B.Q.; Xing, S.H. Short term effects of biochar with different particle sizes on phosphorous availability and microbial communities. Chemosphere Environ. Toxicol. Risk Assess. 2020, 256, 126862.1–126862.15. [Google Scholar] [CrossRef]
- Tian, L.; Liu, J.H.; Zhao, B.P.; Mi, J.Z.; Li, Y.H.; Fei, N. Effects of the combination of super absorbent polymer and microbial fertilizer on soil physical and chemical properties and oat yield in dry farmland. Soils Fertil. Sci. China 2021, 4, 109–117. [Google Scholar]
- Wang, Y.P.; Li, P.; Wu, W.Q.; Jin, Q.; Wang, R.; Zhang, L.; Gao, F.; Zhang, Y.; Wang, W.R. Effects of Bio-Organic Fertilizer and Microbial Agent on the Growth of Tea Chrysanthemum and Soil Fertility under Continuous Cropping Cultivation System in the Mountainous Area of Beijing. Soil Fertil. Sci. China 2023, 10, 1–10. [Google Scholar]
- Zhang, F.; Wang, R.P.; Liang, J.W.; Wu, Y.G.; Wang, X.J.; Liao, X.R. Vermicompost application accelerates organic phosphorus mineralization in a paddy soil: A mechanism study. Ecol. Environ. Sci. 2019, 28, 506–513. [Google Scholar]
- Zhang, W.L.; Xu, A.G.; Zhang, R.L.; Ji, H.J. Review of Soil Classifcation and Revision of China Soil Classifcation System. Sci. Agric. Sin. 2014, 47, 3214–3230. [Google Scholar] [CrossRef]
- DB12/T 1142-2022; Classification Diagnosis of Main Indexes of Cultivated land Fertility. Tianjin Market Supervision and Administration Commission: Tianjin, China, 2022.
- Hou, J.W.; Xing, C.F.; Zhang, J.; Wu, Q.; Zhang, T.T.; Liang, J.M.; An, H.; Lan, H.Q.; Duan, Y. Exogenous organic matter improves potato yield by regulating the microbiological fertility index. Agronomy 2024, 14, 571. [Google Scholar] [CrossRef]
- Siebielec, S.; Grzadziel, M.A.; Siebielec, G.; Jaruga, U.A.; Koziel, M.; Galazka, A.; Przybys, A.; Sugier, P.; Urbniak, M. Microbial Community Response to Various Types of Exogenous Organic Matter Applied to Soil. Int. J. Mol. Sci. 2023, 24, 14559. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wen, J.; Zhang, T.; Zhang, Y.; Peng, Z.; Tang, C.C.; Wang, Y.N.; Su, S.M.; Zhang, N.; Zeng, X.B. Effects of Five-Year Inorganic and Organic Fertilization on Soil Phosphorus Availability and Phosphorus Resupply for Plant P Uptake during Maize Growth. Agriculture 2023, 13, 858. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Yuan, J.H.; Zhu, W.B.; Wang, S.Q. The effect of phosphorus-reduction on soil phosphorus supply and wheat phosphorus uptake in a rice-wheat rotation system in the Taihu Lake Regio. J. Agro-Environ. Sci. 2018, 37, 741–746. [Google Scholar]
- Liu, J.; Yang, J.J. Molecular speciation of phosphorus in agricultural soils: Advances over the last 30 years. Acta Pedol. Sin. 2021, 58, 558–567. [Google Scholar]
- Zhang, H.Z.; Chen, C.R.; Gray, M.E.; Boyd, S.E.; Yang, H.; Zhang, D.K. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Su, Q.; Hou, Z.A.; Zhao, L.; Shu, S.B.; Zhai, Y.; Dong, T.Y. Effects of biochar on soil phosphorus content and cotton nutrient uptake. J. Plant Nutr. Fertil. 2014, 20, 642–650. [Google Scholar]
- Kumar Sootahar, M.; Zeng, X.B.; Wang, Y.N.; Su, S.M.; Soothar, P.; Bai, L.Y.; Kymar, M.; Zhang, Y.; Mustafa, A.; Ye, N. The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures. Plants 2020, 9, 205. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, G.L.; Chang, F.D.; Zhang, H.Y.; Pang, H.C.; Zhang, J.L.; Wang, J.; Ji, H.J.; Li, Y.Y. Effects of microbial agents on physicochemical properties and microbial flora of rhizosphere saline-alkali soil. Ecol. Environ. Sci. 2022, 31, 1984–1992. [Google Scholar]
- Gao, T.Y.; Li, N.; Peng, J.; Gao, M.H.; Luo, P.Y.; Han, X.R. Effect of consecutive application of biochar on phosphate morphology and availability in brown soil. J. Plant Nutr. Fertil. 2019, 25, 1451–1460. [Google Scholar]
- Feng, Y.Y.; Luo, Y.; He, Q.P.; Zhao, D.; Zhang, K.Q.; Shen, S.Z.; Wang, F. Performance and mechanism of a biochar-based Ca-La composite for the adsorption of phosphate from water. J. Environ. Chem. Eng. 2021, 9, 105267. [Google Scholar] [CrossRef]
- Tang, X.L.; Li, Z.J.; Ma, Y.; Liang, Y.C. Mechanism of fulvic acid- and organic manure-mediated phosphorus mobilization in black soil at low temperature. J. Environ. Chem. Eng. 2012, 18, 893–899. [Google Scholar]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Wang, T.; Kong, L.Y.; Jiao, J.G.; Liu, M.Q.; Hu, F.; Sun, B.; Li, H.X. Screeing of phosphate-solubilizing bacteria in red soil and their acting mechanisms. Acta Pedol. Sin. 2014, 51, 373–380. [Google Scholar]
- Li, N.; Yang, J.F.; Liu, H.J.; Han, X.R. Response of soil phosphorus to P balance under long-term rotation and fertilization in brown soil. J. Environ. Chem. Eng. 2018, 24, 1697–1703. [Google Scholar]
- Qi, R.S.; Dang, T.H.; Yang, S.Q.; Ma, R.P.; Zhou, L.P. Forms of soil phosphorus and P adsorption in soil under long-term crop rotation and fertilization systems. Acta Pedol. Sin. 2012, 49, 1136–1146. [Google Scholar]
- Li, H.W.; Wu, X.H.; Liu, T.; He, J.S.; Wang, J.; Yan, W.D. Characteristics of soil phosphorus fractions and phosphatases activity in different plantations. Acta Ecol. Sin. 2023, 43, 1257–1266. [Google Scholar]
- Ma, X.F.; Li, H.G.; Zhang, J.L.; Shen, J.B. Spatiotemporal Pattern of acid phosphatase activity in soils cultivated with maize sensing to phosphorus-rich patches. Front. Plant Sci. 2021, 12, 650436. [Google Scholar] [CrossRef]
- Ji, P.F.; Xu, H.; Zhan, X.; Zhu, G.W.; Zou, W.; Zhu, M.Y.; Kang, L.J. Spatial-temporal variations and driving of nitrogen and phosphorus ratios in lakes in the middle and lower reaches of yangtze river. Environ. Sci. 2020, 41, 4030–4041. [Google Scholar]
- Zhang, Y.; Fan, F.L.; Zhou, C.; Ni, J.P.; Xie, D.L. Effects of Crop/Mulberry Intercropping on Surface Nitrogen and Phosphorus Losses in Three Gorges Reservoir Area. Acta Pedol. Sin. 2016, 53, 189–201. [Google Scholar]









| Type | Available Phosphorus | Ca2-P | Ca8-P | Al-P | Fe-P | O-P | Ca10-P | Neutral Phosphatase | Alkaline Phosphatase | Acid Phosphatase |
|---|---|---|---|---|---|---|---|---|---|---|
| Available phosphorus | 1 | |||||||||
| Ca2-P | 0.697 ** | 1 | ||||||||
| Ca8-P | 0.676 ** | 0.714 ** | 1 | |||||||
| Al-P | 0.667 ** | 0.627 ** | 0.288 | 1 | ||||||
| Fe-P | 0.678 ** | 0.512 ** | 0.579 ** | 0.664 ** | 1 | |||||
| O-P | 0.209 | 0.458 ** | 0.415 ** | −0.243 | −0.110 | 1 | ||||
| Ca10-P | −0.257 | 0.018 | −0.124 | −0.409 ** | −0.434 ** | 0.674 ** | 1 | |||
| Neutral phosphatase | 0.717 ** | 0.820 ** | 0.845 ** | 0.346 * | 0.474 ** | 0.584 ** | 0.016 | 1 | ||
| Alkaline phosphatase | −0.070 | −0.067 | −0.221 | 0.103 | −0.071 | 0.071 | 0.140 | −0.213 | 1 | |
| Acid phosphatase | 0.685 ** | 0.826 ** | 0.858 ** | 0.470 ** | 0.520 ** | 0.362 * | −0.157 | 0.922 ** | −0.274 | 1 |
| Soil Depth (cm) | Organic Matter (g·kg−1) | Total Nitrogen (g·kg−1) | Alkaline Hydrolyzable Nitrogen (mg·kg−1) | Available Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) | pH |
|---|---|---|---|---|---|---|
| 0–20 | 18.57 | 0.49 | 83.84 | 40.18 | 154.5 | 7.10 |
| 20–40 | 10.27 | 0.30 | 55.57 | 18.89 | 121.3 |
| Treatment Code | Treatment |
|---|---|
| CK | conventional fertilization (other treatments were added based on CK) |
| T1 | fulvic acid (59.97 kg·hm−2) |
| T2 | biochar (900 kg·hm−2) |
| T3 | compound microbial fertilizer (748 kg·hm−2) |
| T4 | high-energy microbial inoculum (748 kg·hm−2) |
| T5 | pig manure-vermicompost (4000 kg·hm−2) |
| T6 | cow manure-vermicompost (4000 kg·hm−2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Gu, D.; Yang, H.; Li, Y.; Jiang, Y.; Zhan, N.; Cui, X. Effect of Exogenous Organic Matter on Phosphorus Forms in Middle-High Fertility Cinnamon Soil. Plants 2024, 13, 1313. https://doi.org/10.3390/plants13101313
Shi X, Gu D, Yang H, Li Y, Jiang Y, Zhan N, Cui X. Effect of Exogenous Organic Matter on Phosphorus Forms in Middle-High Fertility Cinnamon Soil. Plants. 2024; 13(10):1313. https://doi.org/10.3390/plants13101313
Chicago/Turabian StyleShi, Xiaodi, Duanyin Gu, Haotian Yang, Yun Li, Yaqun Jiang, Nanbiao Zhan, and Xiumin Cui. 2024. "Effect of Exogenous Organic Matter on Phosphorus Forms in Middle-High Fertility Cinnamon Soil" Plants 13, no. 10: 1313. https://doi.org/10.3390/plants13101313





