Synthesis, Spectral Characteristics, Sensing Properties and Microbiological Activity of New Water-Soluble 4-Sulfo-1,8-naphthalimides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Synthesis of Potassium 2-(2-Acetamidoethyl)-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinoline-6-sulfonate MSNI
2.3. Synthesis of 4-Sulfo-1,8-naphthalimide Based Dendrimer DSNI
2.4. Synthesis of Cu(II) Complex of Dendrimer DSNI: [Cu2(DSNI)(NO3)4]
2.5. Treatment of Cotton Fabric with MSNI, DSNI, and [Cu2(DSNI)(NO3)4]
3. Results and Discussion
3.1. Synthesis of 4-Sulfo-1,8-naphthalimides: MSNI, DSNI and [Cu2(DSNI)(NO3)4]
3.2. Photophysical Properties of MSNI and DSNI
3.3. Effect of pH on the Fluorescence Intensity of MSNI and DSNI
3.4. FTIR Characterization of [Cu2(DSNI)(NO3)4]
3.5. EPR Analysis of the Complex of Cu2+ with DSNI Ligand [Cu2(DSNI)(NO3)4]
3.6. The Effect of Light on Bacterial Growth
3.7. Antimicrobial Activity of Treated Cotton Fabrics
3.8. Cytotoxicity and Virucidal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisi, N.; Pinzi, L.; Rastelli, G.; Tonali, N. Early Diagnosis of Neurodegenerative Diseases: What Has Been Undertaken to Promote the Transition from PET to Fluorescence Tracers. Molecules 2024, 29, 722. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, S.M.; Kim, H.Y.; Kim, Y. Fluorescence Chemicals to Detect Insoluble and Soluble Amyloid-β Aggregates. ACS Chem. Neurosci. 2019, 10, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Blazevicius, D.; Grigalevicius, S. A Review of Benzophenone-Based Derivatives for Organic Light-Emitting Diodes. Nanomaterials 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Radotić, K.; Stanković, M.; Bartolić, D.; Natić, M. Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach. Foods 2023, 12, 3023. [Google Scholar] [CrossRef] [PubMed]
- Dodangeh, M.; Grabchev, I.; Staneva, D.; Gharanjig, K. 1,8-Naphthalimide Derivatives as Dyes for Textile and Polymeric Materials: A Review. Fibers Polym. 2021, 22, 2368–2379. [Google Scholar] [CrossRef]
- Yang, J.-X.; Wang, X.-L.; Tusong; Xu, L.-H. Studies on the Synthesis and Spectral Properties of Novel 4-Benzofuranyl-1,8-Naphthalimide Derivatives. Dyes Pigments 2005, 67, 27–33. [Google Scholar] [CrossRef]
- Poteau, X.; Brown, A.I.; Brown, R.G.; Holmes, C.; Matthew, D. Fluorescence Switching in 4-Amino-1,8-Naphthalimides: “On–off–on” Operation Controlled by Solvent and Cations. Dyes Pigments 2000, 47, 91–105. [Google Scholar] [CrossRef]
- Grabchev, I.; Angelova, S.; Staneva, D. Yellow-Green and Blue Fluorescent 1,8-Naphthalimide-Based Chemosensors for Metal Cations. Inorganics 2023, 11, 47. [Google Scholar] [CrossRef]
- Agafontsev, A.M.; Oshchepkov, A.S.; Shumilova, T.A.; Kataev, E.A. Binding and Sensing Properties of a Hybrid Naphthalimide–Pyrene Aza-Cyclophane towards Nucleotides in an Aqueous Solution. Molecules 2021, 26, 980. [Google Scholar] [CrossRef]
- Haque, A.; Alenezi, K.M.; Al-Otaibi, A.; Alsukaibi, A.K.D.; Rahman, A.; Hsieh, M.-F.; Tseng, M.-W.; Wong, W.-Y. Synthesis, Characterization, Cytotoxicity, Cellular Imaging, Molecular Docking, and ADMET Studies of Piperazine-Linked 1,8-Naphthalimide-Arylsulfonyl Derivatives. Int. J. Mol. Sci. 2024, 25, 1069. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Bakov, V.V.; Anichina, K.K.; Bojinov, V.B. Fluorescent Probes as a Tool in Diagnostic and Drug Delivery Systems. Pharmaceuticals 2023, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Gopala, L.; Cha, Y.; Lee, M.H. Versatile naphthalimides: Their optical and biological behavior and applications from sensing to therapeutic purposes. Dyes Pigments 2022, 201, 110195. [Google Scholar] [CrossRef]
- Ingrassia, L.; LeFranc, F.; Kiss, R.; Mijatovic, T. Naphthalimides and Azonafides as Promising Anti-Cancer Agents. Curr. Med. Chem. 2009, 16, 1192–1213. [Google Scholar] [CrossRef]
- Gong, H.H.; Addla, D.; Lv, J.S.; Zhou, C.H. Heterocyclic naphthalimides as new skeleton structure of compounds with increasingly expanding relational medicinal applications. Curr. Top. Med. Chem. 2016, 16, 3303–3364. [Google Scholar] [CrossRef] [PubMed]
- Carretero, G.P.B.; Saraiva, G.K.V.; Rodrigues, M.A.; Kiyota, S.; Bemquerer, M.P.; Chaimovich, H.; Cuccovia, I.M. Naphthalimide-Containing BP100 Leads to Higher Model Membranes Interactions and Antimicrobial Activity. Biomolecules 2021, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Dutta, T.; Nema, S.; Koner, A.L. Detection of Lysosomal Hg2+ Using a pH-Independent Naphthalene Monoimide-Based Fluoroprobe. Chemosensors 2023, 11, 184. [Google Scholar] [CrossRef]
- Geraghty, C.; Wynne, C.; Elmes, R.B.P. 1,8-Naphthalimide based fluorescent sensors for enzymes. Coord. Chem. Rev. 2021, 437, 213713. [Google Scholar] [CrossRef]
- Han, C.; Sun, S.-B.; Ji, X.; Wang, J.-Y. Recent advances in 1,8-naphthalimide-based responsive small-molecule fluorescent probes with a modified C4 position for the detection of biomolecules. Trends Anal. Chem. 2023, 167, 117242. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Lan, Y.; Yan, M. Design strategies of logic gate sensors based on FRET mechanism. Trends Anal. Chem. 2023, 167, 117271. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Fedorova, O.A.; Fedorov, Y.V. Fluorescent and colorimetric chemosensors for cations based on 1,8-naphthalimide derivatives: Design principles and optical signalling mechanisms. Russ. Chem. Rev. 2014, 83, 155–182. [Google Scholar] [CrossRef]
- Pooresmaeila, M.; Namaz, H. Advances in development of the dendrimers having natural saccharides in their structure for efficient and controlled drug delivery applications. Eur. Polym. J. 2021, 148, 110356. [Google Scholar] [CrossRef]
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Parveen, F.; Torchilin, V. Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases. Molecules 2021, 26, 3304. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Kaluzna-Mlynarczyk, A.; Goslinski, T. Dendrimers against fungi—A state of the art review. J. Control. Release 2021, 330, 599–617. [Google Scholar] [CrossRef]
- Tarach, P.; Janaszewska, A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I. Chapter 20, Dendrimer as antimicrobial agents. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 363–384. [Google Scholar] [CrossRef]
- Nandhikonda, P.; Begaye, M.P.; Cao, Z.; Heagy, M.D. Frontier molecular orbital analysis of dual fluorescent dyes: Predicting two-color emission in N-Aryl-1,8-naphthalimides. Org. Biomol. Chem. 2010, 8, 3195–3201. [Google Scholar] [CrossRef]
- Wang, B.-B.; Zhang, X.; Jia, X.-R.; Li, Z.-C.; Ji, Y.; Yang, L.; Wei, Y. Fluorescence and Aggregation Behavior of Poly (Amidoamine) Dendrimers Peripherally Modified with Aromatic Chromophores: The Effect of Dendritic Architectures. J. Am. Chem. Soc. 2004, 126, 15180–15194. [Google Scholar] [CrossRef]
- Petasis, D.T.; Hendrich, M.P. Quantitative Interpretation of Multifrequency Multimode EPR Spectra of Metal Containing Proteins, Enzymes, and Biomimetic Complexes. Methods Enzymol. 2015, 563, 171–208. [Google Scholar] [CrossRef]
- Grabchev, I.; Vasileva-Tonkova, E.; Staneva, D.; Bosch, P.; Kukeva, R.; Stoyanova, R. Synthesis, spectral characterization, and in vitro antimicrobial activity in liquid medium and applied on cotton fabric of a new PAMAM metallodendrimer. Int. J. Polym. Anal. Charact. 2018, 23, 45–57. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Tomé, J.P.C. Photodynamic inactivation of bacteria: Finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef]

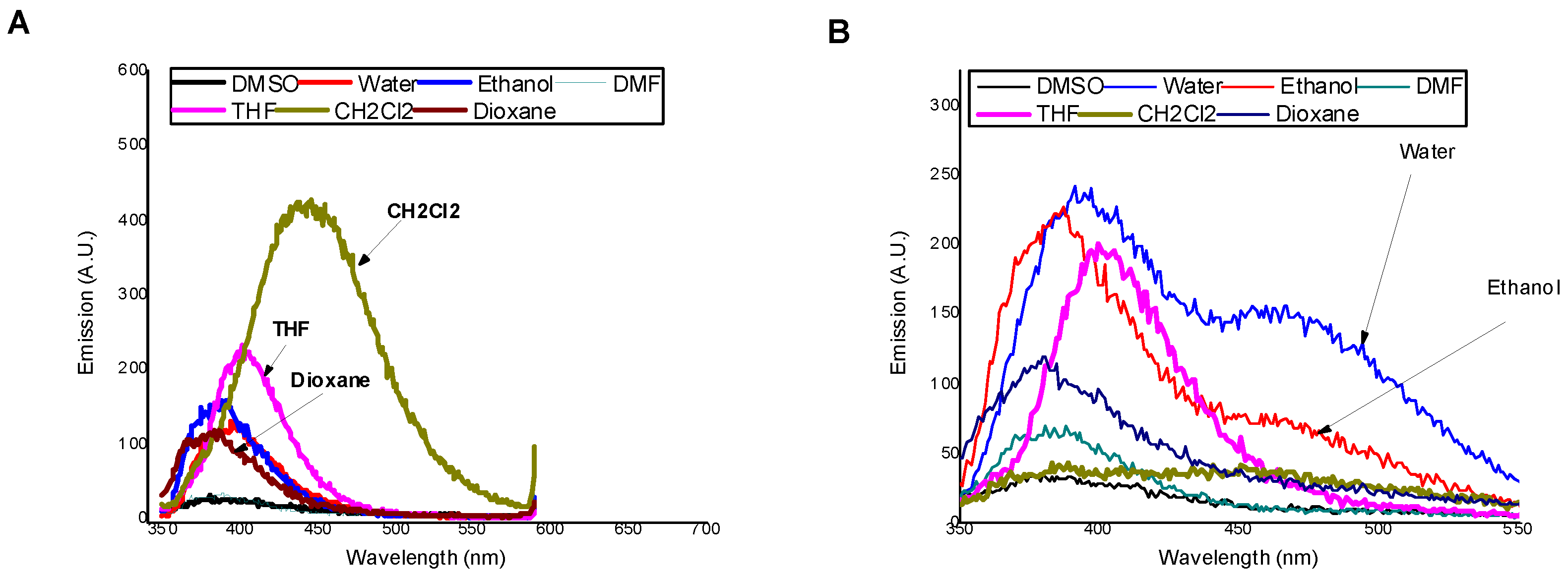
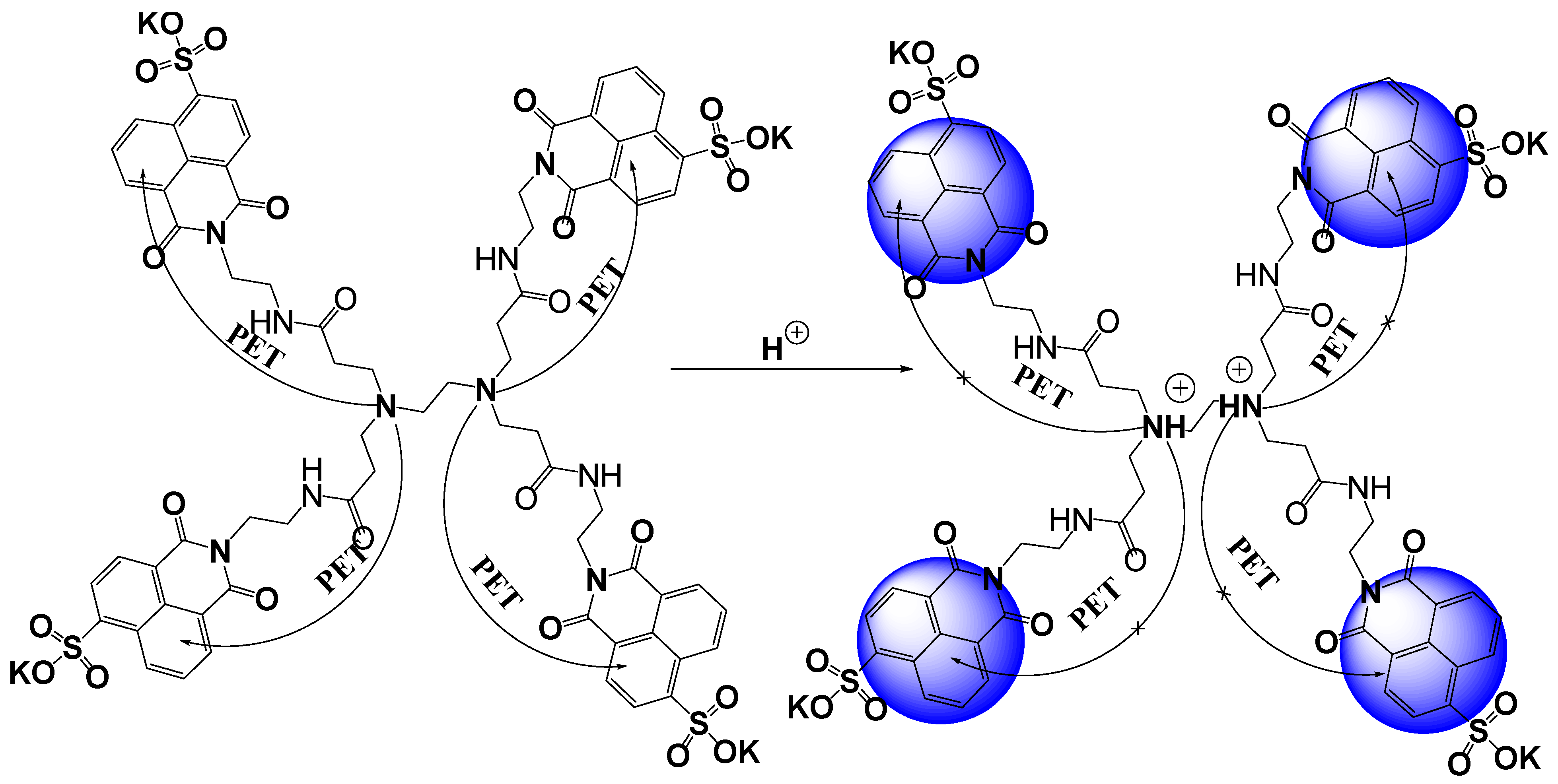






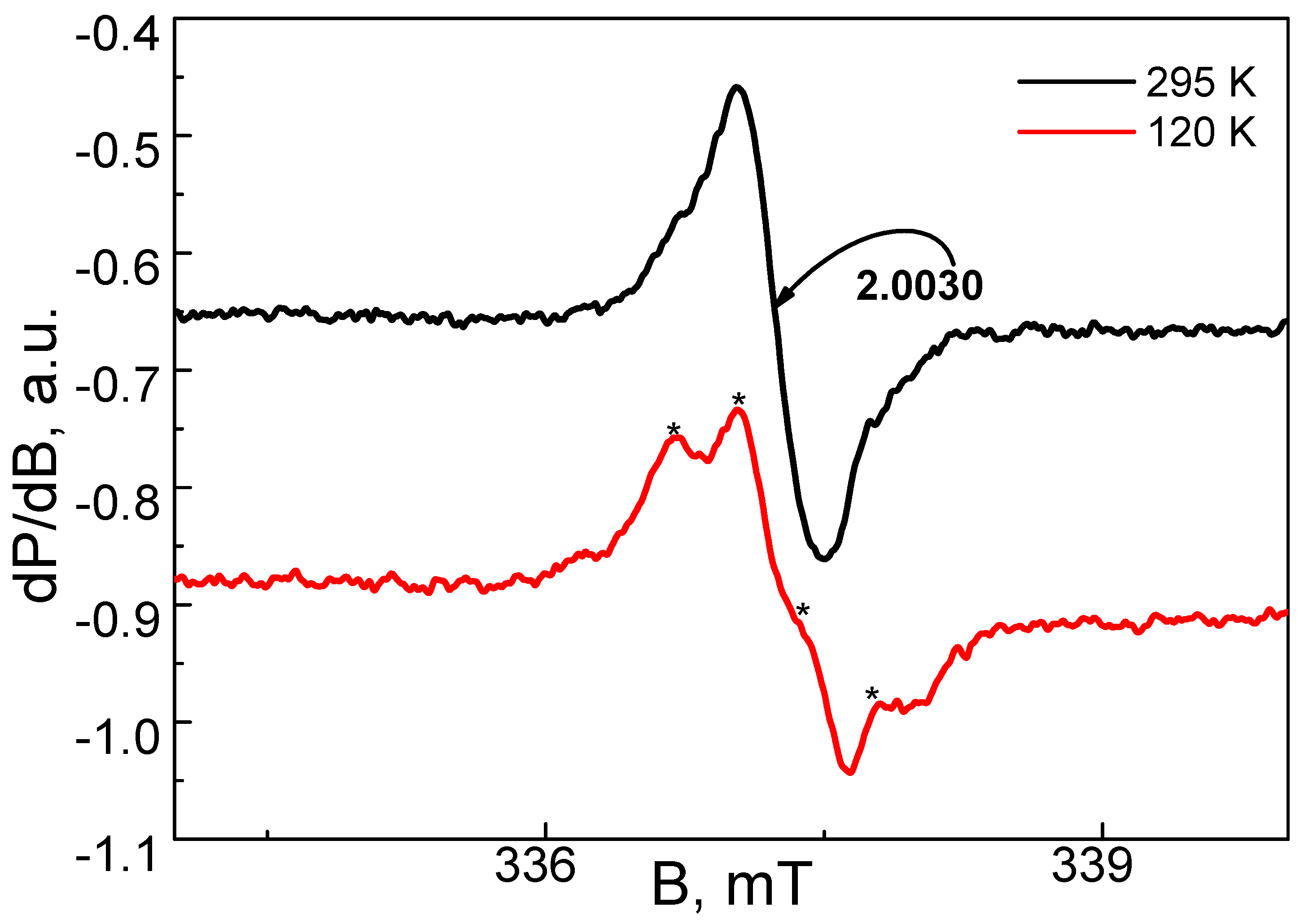



| Solvents | Water | DMSO | DMF | Ethanol | CH2Cl2 | THF | Dioxane |
|---|---|---|---|---|---|---|---|
| MSNI | |||||||
| Dielectric constant (25 °C) | 78.35 | 47.1 | 37.1 | 24.5 | 8.93 | 7.58 | 2.25 |
| λabs. (nm) | 338, 351 | 342, 357 | 342, 357 | 336, 352 | 339 | 340, 355 | 337, 354 |
| λem. (nm) | 394 | 390 | 388 | 387 | 444 | 401 | 367 |
| Stockes shift (cm−1) | 3109 | 2370 | 2238 | 2569 | 6976 | 3223 | 1081 |
| ε (L mol−1cm−1) | 10,200 9800 | 13,700 12,500 | 8900 13,300 12,000 | 9200 11,700 10,500 | 8200 7300 | 13,600 12,000 | 6900 9300 8600 |
| ΦF | 0.035 | 0.004 | 0.002 | 0.005 | 0.080 | 0.017 | 0.005 |
| DSNI | |||||||
| λabs. (nm) | 342 | 341, 356 | 341, 357 | 337 | 340 | 340 | 341 |
| λem. (nm) | 396,465 | 386 | 382 | 463 | 387,459 | 402 | 381 |
| Stockes shift (cm−1) | 6615 | 2183 | 1833 | 8075 | 7325 | 4536 | 3079 |
| ε (L mol−1cm−1) | 40,400 | 67,700 56,500 | 59,800 41,400 | 49,400 | 48,600 40,400 | 60,300 | 41900 |
| ΦF | 0.060 | 0.002 | 0.002 | 0.0035 | - | 0.009 | 0.007 |
| Virus | Δlog 30 min | ||
| MSNI | DSNI | [Cu2(DSNI)(NO3)4] | |
| HRSV-2 | 0.9 | 1 | 0.8 |
| Virus | Δlog 60 min | ||
| MSNI | DSNI | [Cu2(DSNI)(NO3)4] | |
| HRSV-2 | 1.2 | 1.1 | 1 |
| Virus | Δlog 30 min | ||
| MSNI | DSNI | [Cu2(DSNI)(NO3)4] | |
| HRSV-2 | 0.8 | 0.9 | 0.6 |
| Virus | Δlog 60 min | ||
| MSNI | DSNI | [Cu2(DSNI)(NO3)4] | |
| HRSV-2 | 1 | 1 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, A.I.; Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Stoyanova, R.; Jordanova, A.; Grabchev, I. Synthesis, Spectral Characteristics, Sensing Properties and Microbiological Activity of New Water-Soluble 4-Sulfo-1,8-naphthalimides. Chemosensors 2024, 12, 79. https://doi.org/10.3390/chemosensors12050079
Said AI, Staneva D, Vasileva-Tonkova E, Grozdanov P, Nikolova I, Stoyanova R, Jordanova A, Grabchev I. Synthesis, Spectral Characteristics, Sensing Properties and Microbiological Activity of New Water-Soluble 4-Sulfo-1,8-naphthalimides. Chemosensors. 2024; 12(5):79. https://doi.org/10.3390/chemosensors12050079
Chicago/Turabian StyleSaid, Awad I., Desislava Staneva, Evgenia Vasileva-Tonkova, Petar Grozdanov, Ivanka Nikolova, Radostina Stoyanova, Albena Jordanova, and Ivo Grabchev. 2024. "Synthesis, Spectral Characteristics, Sensing Properties and Microbiological Activity of New Water-Soluble 4-Sulfo-1,8-naphthalimides" Chemosensors 12, no. 5: 79. https://doi.org/10.3390/chemosensors12050079







