Synergistic Antinociceptive Effect of β-Caryophyllene Oxide in Combination with Paracetamol, and the Corresponding Gastroprotective Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Anti-Nociceptive Evaluation
2.4. Evaluation of the Type of Interaction of the Compounds in the Combination Treatment
2.5. Evaluation of the Mechanism of Action of β-Caryophyllene Oxide
2.6. Determination of the Protective Effect of the Combination Treatment on the Gastric Mucosa
2.7. Statistical Analysis
3. Results
3.1. Anti-Nociceptive Effect of Paracetamol and β-Caryophyllene Oxide
3.2. Anti-Nociceptive Effect of the Combination Treatment
3.3. Mechanism of Action of β-Caryophyllene Oxide
3.3.1. Participation of Opioid Receptors
3.3.2. Contribution of Serotonin Receptors
3.3.3. Participation of l-Arginine
3.3.4. Contribution of NO
3.3.5. Participation of cGMP
3.3.6. Contribution of the ATP-Sensitive K+ Channel
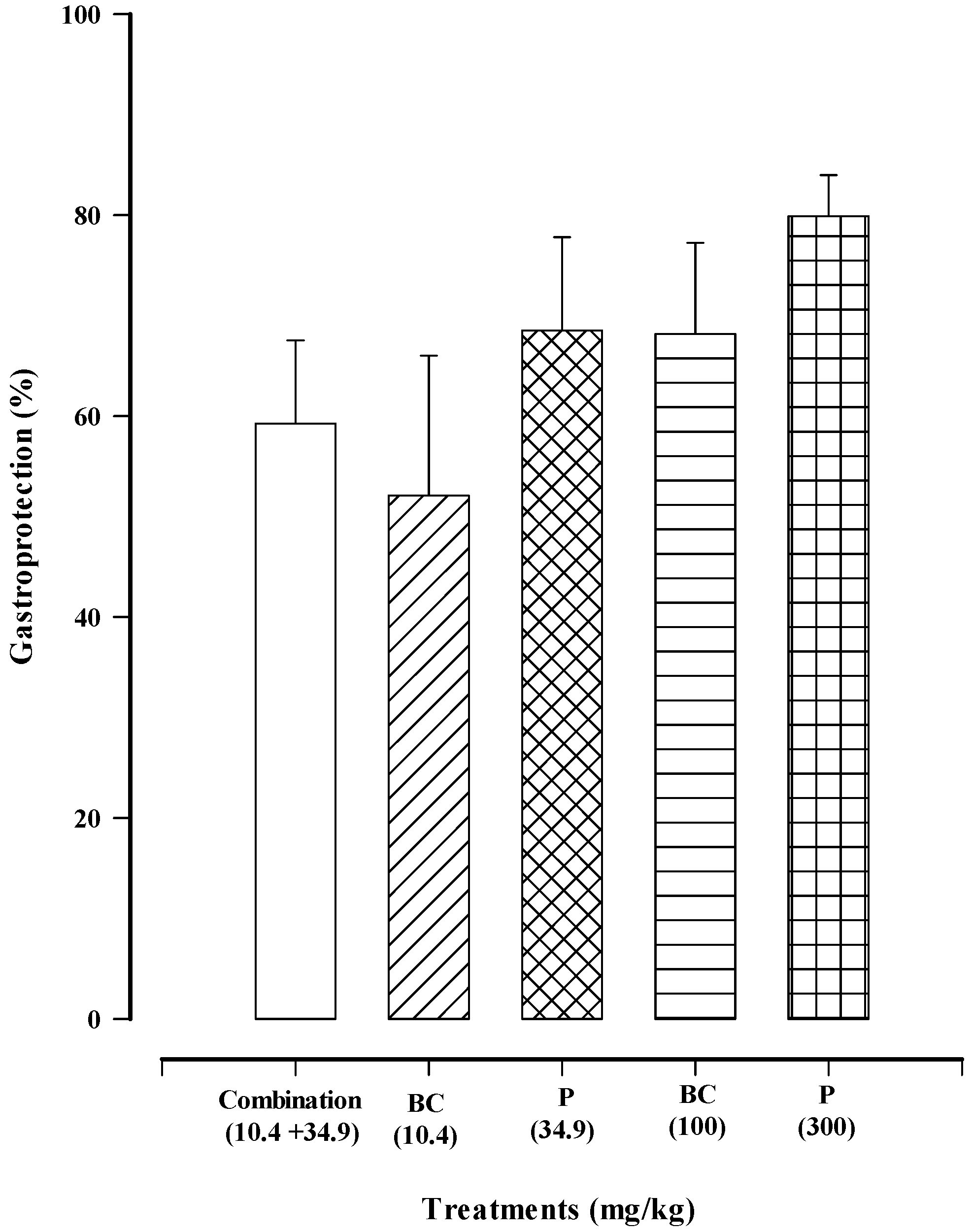
3.3.7. The Effect of the Combination Treatment (Paracetamol + β-Caryophyllene Oxide) on the Gastric Mucosa
4. Discussion
5. Future Prospects
6. Study Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Association for the Study of Pain, Terminology. Available online: https://www.iasp-pain.org/resources/terminology/#pain (accessed on 10 October 2023).
- Glare, P.; Overton, S.; Aubrey, K. Transition from acute to chronic pain: Where cells, systems and society meet. Pain Manag. 2020, 10, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Marcianò, G.; Vocca, C.; Evangelista, M.; Palleria, C.; Muraca, L.; Galati, C.; Monea, F.; Sportiello, L.; De Sarro, G.; Capuano, A.; et al. The Pharmacological Treatment of Chronic Pain: From Guidelines to Daily Clinical Practice. Pharmaceutics 2023, 15, 1165. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Amaechi, O.; Huffman, M.M.; Featherstone, K. Pharmacologic Therapy for Acute Pain. Am. Fam. Physician 2021, 104, 63–72. [Google Scholar] [PubMed]
- Dale, R.; Stacey, B. Multimodal Treatment of Chronic Pain. Med. Clin. N. Am. 2016, 100, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Silvagni, D.; Chiarugi, A.; Cortis, E.; D’Avino, A.; Lanari, M.; Marchisio, P.G.; Vezzoli, C.; Zampogna, S.; Staiano, A. Paracetamol and ibuprofen combination for the management of acute mild-to-moderate pain in children: Expert consensus using the Nominal Group Technique (NGT). Ital. J. Pediatr. 2023, 49, 36. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Kondo, Y.; Kadowaki, D. Toxicological Property of Acetaminophen: The Dark Side of a Safe Antipyretic/Analgesic Drug? Biol. Pharm. Bull. 2020, 43, 195–206. [Google Scholar] [CrossRef]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2021, 48, 3–19. [Google Scholar] [CrossRef]
- Ohashi, N.; Kohno, T. Analgesic Effect of Acetaminophen: A Review of Known and Novel Mechanisms of Action. Front. Pharmacol. 2020, 11, 580289. [Google Scholar] [CrossRef]
- WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents, Geneva: World Health Organization. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537492/ (accessed on 10 October 2023).
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Zapata-Morales, J.R.; Solorio-Alvarado, C.; Hernández-Santiago, A.; Espinoza-Ramírez, L.A.; Carranza-Álvarez, C.; Ramadoss, V. Central nervous system evaluation of an ethanol extract of Bidens odorata Cav (Asteraceae) leaves, and its antinociceptive interaction with paracetamol and naproxen. Inflammopharmacology 2020, 28, 749–757. [Google Scholar] [CrossRef]
- Lazar, A.; Petar, M.; Drago, A.; Petar, N.; Spasoje, V.; Stojšić-Milosavljević, A.Đ.; Paut, M.N.; Branko, D. Antinociceptive activity of Thyme (Thymus vulgaris L.) and interactions with neurotropics and analgesics. Braz. J. Pharm. Sci. 2020, 56, e18819. [Google Scholar]
- Oppong-Damoah, A.; Blough, B.E.; Makriyannis, A.; Murnane, K.S. The sesquiterpene beta-caryophyllene oxide attenuates ethanol drinking and place conditioning in mice. Heliyon 2019, 5, e01915. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Técnicas para la Producción, Cuidado y uso de los Animals de Laboratorio. Diario Oficial de la Federación. Norma Oficial Mexicana: Mexico City, Mexico, 1999.
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- Tallarida, R.J.; Murray, R.B. Area under a curve: Trapezoidal and Simpson’s rules. In Manual of Pharmacologic Calculations; Springer: London, UK, 1987; pp. 77–81. [Google Scholar]
- Tallarida, R.J. Drug Synergism and Dose-Effect Data Analysis, 1st ed.; Chapman & Hall/CRC: New York, NY, USA, 2000; pp. 57–89. [Google Scholar]
- Tallarida, R.J. The Interaction Index: A Measure of Drug Synergism. Pain 2002, 98, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.K.; Oluyomi, A.O.; Babbedge, R.C.; Wallace, P.; Hart, S.L. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br. J. Pharmacol. 1991, 102, 98–202. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Bautista, M.; Velázquez-González, C.; De la O-Arciniega, M.; González-Olivares, L.G.; Fernández-Moya, M.; Jaramillo-Morales, O.A. Antinociceptive Synergism of Pomegranate Peel Extract and Acetylsalicylic Acid in an Animal Pain Model. Molecules 2021, 26, 5434. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Silverio, J.; Sánchez-Mendoza, M.E.; Rocha-González, H.I.; Reyes-García, J.G.; Flores-Murrieta, F.J.; López-Lorenzo, Y.; Quiñonez-Bastidas, G.N.; Arrieta, J. Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid. Molecules 2021, 26, 2921. [Google Scholar] [CrossRef]
- Vargas-Ruiz, R.; Roa-Coria, J.E.; Mora-Olivo, A.; Zamilpa, A.; Herrera-Ruiz, M.L.; Acosta-González, R.I.; Montiel-Ruiz, R.M. Antinociceptive and anti-inflammatory effect of a standardized fraction of Oenothera rosea L’Hér. ex Aiton and its possible mechanism of action in mice. Avicenna J. Phytomed. 2022, 12, 401–413. [Google Scholar]
- Sánchez-Mendoza, M.E.; López-Lorenzo, Y.; Cruz-Antonio, L.; Cruz-Oseguera, A.; García-Machorro, J.; Arrieta, J. Gastroprotective Effect of Juanislamin on Ethanol-Induced Gastric Lesions in Rats: Role of Prostaglandins, Nitric Oxide and Sulfhydryl Groups in the Mechanism of Action. Molecules 2020, 25, 2246. [Google Scholar] [CrossRef]
- Beltrán-Villalobos, K.L.; Déciga-Campos, M.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Martínez-Salazar, M.F.; Ramírez-Cisneros, M.A.; Rios, M.Y. Synergistic antinociceptive interaction of Syzygium aromaticum or Rosmarinus officinalis coadministered with ketorolac in rats. Biomed. Pharmacother. 2017, 94, 858–864. [Google Scholar] [CrossRef]
- Högestätt, E.D.; Jönsson, B.A.G.; Ermund, A.; Andersson, D.A.; Björk, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef]
- Barrot, M. Tests and models of nociception and pain in rodents. Neuroscience 2012, 211, 39–50. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Parseghyan, L.; Sevoyan, G.; Darbinyan, A.; Sahakyan, N.; Gaboyan, M.; Karabekian, Z.; Voskanyan, A. Antinociceptive, anti-inflammatory, and cytotoxic properties of Origanum vulgare essential oil, rich with β-caryophyllene and β-caryophyllene oxide. Korean J. Pain 2022, 35, 140–151. [Google Scholar] [CrossRef]
- Noriega, V.; Miranda, H.F.; Prieto, J.C.; Sotomayor-Zárate, R.; Sierralta, F. Involvement of NO in Antinociception of NSAIDS in Murine Formalin Hind Paw Assay. Drug Res. 2020, 70, 145–150. [Google Scholar] [CrossRef]
- Gelgor, L.; Cartmell, S.; Mitchell, D. Intracerebroventricular microinjections of non-steroidal anti-inflammatory drugs abolish reperfusion hyperalgesia in the rat’s tail. Pain 1992, 50, 323–329. [Google Scholar] [CrossRef]
- Bannwarth, B.; Netter, P.; Lapicque, F.; Gillet, P.; Péré, P.; Boccard, E.; Royer, R.J.; Gaucher, A. Plasma and cerebrospinal fluid concentrations of paracetamol after a single intravenous dose of propacetamol. Br. J. Clin. Pharmacol. 1992, 34, 79–81. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Z.; Lin, J.; Yang, Y.; Ran, T.; Chen, H. The recent progress of deep-learning-based in silico prediction of drug combination. Drug Discov. Today 2023, 28, 103625. [Google Scholar] [CrossRef]
- Stein, C. New concepts in opioid analgesia. Expert Opin. Investig. Drugs 2018, 27, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Hamurtekin, Y.; Nouilati, A.; Demirbatir, C.; Hamurtekin, E. The Contribution of Serotonergic Receptors and Nitric Oxide Systems in the Analgesic Effect of Acetaminophen: An Overview of the Last Decade. Turk. J. Pharm. Sci. 2020, 17, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Gao, S.J.; Sun, J.; Zhang, L.Q.; Wu, J.Y.; Song, F.H.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain. Neural Regen. Res. 2023, 18, 996–1003. [Google Scholar] [PubMed]
- Xia, H.; Zhang, D.; Yang, S.; Wang, Y.; Xu, L.; Wu, J.; Ren, J.; Yao, W.; Fan, L.; Zhang, C.; et al. Role of ATP-sensitive potassium channels in modulating nociception in rat model of bone cancer pain. Brain Res. 2014, 1554, 29–35. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, M.E.; Cruz-Antonio, L.; Cupido-Sánchez, M.G.; García-Castillo, G.; Arrieta, J. Gastroprotective activity of caryophyllene oxide: The role of nitric oxide, prostaglandins and sulfhydryls. J. Appl. Pharm. Sci. 2014, 4, 1–5. [Google Scholar]
- Konturek, S.J.; Brzozowski, T.; Piastucki, I.; Radecki, T. Prevention of ethanol and aspirin-induced gastric mucosal lesions by paracetamol and salicylate in rats: Role of endogenous prostaglandins. Gut 1982, 23, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Van Kolfschoten, A.A.; Zandberg, P.; Jager, L.P.; Van Noordwijk, J. Protection by paracetamol against various gastric irritants in the rat. Toxicol. Appl. Pharmacol. 1983, 69, 37–42. [Google Scholar] [CrossRef]
- Fukushima, E.; Monoi, N.; Mikoshiba, S.; Hirayama, Y.; Serizawa, T.; Adachi, K.; Koide, M.; Ohdera, M.; Murakoshi, M.; Kato, H. Protective effects of acetaminophen on ibuprofen-induced gastric mucosal damage in rats with associated suppression of matrix metalloproteinase. J. Pharmacol. Exp. Ther. 2014, 349, 165–173. [Google Scholar] [CrossRef]






| Combination | Doses of Paracetamol (mg/kg) | Doses of β-Caryophyllene Oxide (mg/kg) | Total (mg/kg) |
|---|---|---|---|
| 1 | 98.17 | 29.67 | 127.84 |
| 2 | 49.08 | 14.84 | 63.92 |
| 3 | 24.54 | 7.42 | 31.96 |
| 4 | 12.27 | 3.71 | 15.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Juárez, J.V.; Arrieta, J.; Briones-Aranda, A.; Cruz-Antonio, L.; López-Lorenzo, Y.; Sánchez-Mendoza, M.E. Synergistic Antinociceptive Effect of β-Caryophyllene Oxide in Combination with Paracetamol, and the Corresponding Gastroprotective Activity. Biomedicines 2024, 12, 1037. https://doi.org/10.3390/biomedicines12051037
Espinosa-Juárez JV, Arrieta J, Briones-Aranda A, Cruz-Antonio L, López-Lorenzo Y, Sánchez-Mendoza ME. Synergistic Antinociceptive Effect of β-Caryophyllene Oxide in Combination with Paracetamol, and the Corresponding Gastroprotective Activity. Biomedicines. 2024; 12(5):1037. https://doi.org/10.3390/biomedicines12051037
Chicago/Turabian StyleEspinosa-Juárez, Josué Vidal, Jesús Arrieta, Alfredo Briones-Aranda, Leticia Cruz-Antonio, Yaraset López-Lorenzo, and María Elena Sánchez-Mendoza. 2024. "Synergistic Antinociceptive Effect of β-Caryophyllene Oxide in Combination with Paracetamol, and the Corresponding Gastroprotective Activity" Biomedicines 12, no. 5: 1037. https://doi.org/10.3390/biomedicines12051037





