Activity of NAD(P)H-Oxidoreductases in Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Preparation of Tissue and Peritoneal Fluid Samples
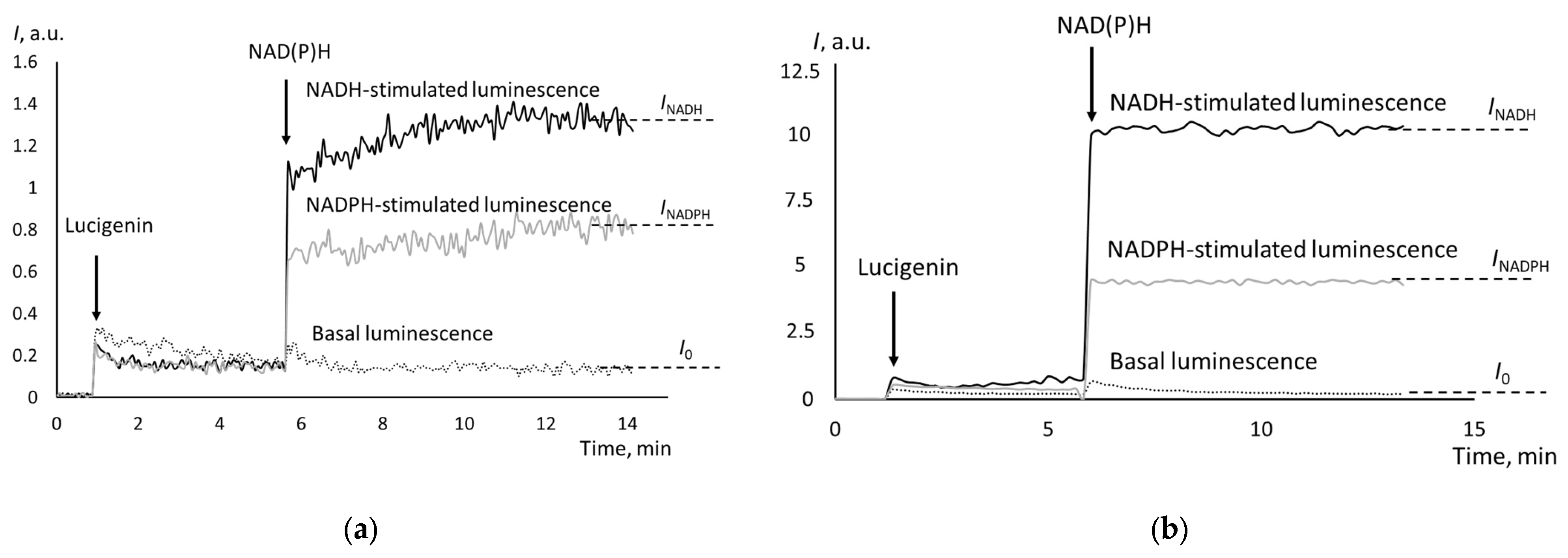
2.3. Assessment of Tissue NAD(P)H Oxidoreductases with Chemiluminescence
2.4. Assessment of NAD(P)H Oxidoreductases in Cells Isolated form Peritoneal Fluid
2.5. Chemiluminescence Assay for Antioxidant Capacity of Peritoneal Fluid
2.6. Chemiluminescence Assay for Studying Effects of Uricase in Antioxidant Capacity
2.7. Statistics
3. Results
3.1. NAD(P)H Oxidoreductases Activity in Ovarian Cancer Tissues
3.2. CYB5R and CYPOR Activity and Chemoresistance
3.3. Antioxidant Capacity of Peritoneal Fluid
3.4. CYB5R and CYPOR Activity in Cells Isolated from Peritoneal Fluid
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Acharya, S.; Karthikeyan, M.; Biswas, P.; Kumari, S. Limitations and potential of immunotherapy in ovarian cancer. Front. Immunol. 2023, 14, 1292166. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Global Cancer Facts & Figures, 3rd ed.; American Cancer Society: Atlanta, GA, USA, 2015. [Google Scholar]
- Lopez-Portugues, C.; Montes-Bayon, M.; Diez, P. Biomarkers in Ovarian Cancer: Towards Personalized Medicine. Proteomes 2024, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Wu, Y. Tumor metabolism rewiring in epithelial ovarian cancer. J. Ovarian Res. 2023, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutoglou, C.; Dampala, K.; Anthoulakis, C.; Papanikolaou, E.G.; Tentas, I.; Dragoutsos, G.; Machairiotis, N.; Zarogoulidis, P.; Ioannidis, A.; Matthaios, D.; et al. Epithelial Ovarian Cancer: A Five Year Review. Medicina 2023, 59, 1183. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhu, X.; Zhong, L.; Jiang, Q.; Wang, Y.; Tang, Q.; Li, Q.; Zhang, C.; Wang, H.; et al. Drug resistance in ovarian cancer: From mechanism to clinical trial. Mol. Cancer 2024, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- El Bairi, K.; Madariaga, A.; Trapani, D.; Al Jarroudi, O.; Afqir, S. New horizons for platinum-resistant ovarian cancer: Insights from the 2023 American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) Annual Meetings. Int. J. Gynecol. Cancer 2023, 34, 760–772. [Google Scholar] [CrossRef]
- Eskander, R.N.; Moore, K.N.; Monk, B.J.; Herzog, T.J.; Annunziata, C.M.; O’Malley, D.M.; Coleman, R.L. Overcoming the challenges of drug development in platinum-resistant ovarian cancer. Front. Oncol. 2023, 13, 1258228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhuang, P.; Chen, Y.; Wu, Y.; Zhong, M.; Lun, Y. “Double-edged sword” effect of reactive oxygen species (ROS) in tumor development and carcinogenesis. Physiol. Res. 2023, 72, 301–307. [Google Scholar] [CrossRef]
- Saed, G.M.; Diamond, M.P.; Fletcher, N.M. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.N.; Xie, L.Z.; Shen, Y.; Li, J.; Guo, Y.; Fu, Y.; Liu, F.Y.; Han, F.J. Insights into the Role of Oxidative Stress in Ovarian Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 8388258. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Guo, E.; Zhou, B.; Shan, W.; Huang, J.; Weng, D.; Wu, P.; Wang, C.; Wang, S.; Zhang, W.; et al. A reactive oxygen species scoring system predicts cisplatin sensitivity and prognosis in ovarian cancer patients. BMC Cancer 2019, 19, 1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Huang, L.; Du, Y.; Gan, F.; Li, Y.; Yao, Y. Antioxidative Stress: Inhibiting Reactive Oxygen Species Production as a Cause of Radioresistance and Chemoresistance. Oxid. Med. Cell. Longev. 2021, 2021, 6620306. [Google Scholar] [CrossRef] [PubMed]
- Elahian, F.; Sepehrizadeh, Z.; Moghimi, B.; Mirzaei, S.A. Human cytochrome b5 reductase: Structure, function, and potential applications. Crit. Rev. Biotechnol. 2014, 34, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Lund, R.R.; Leth-Larsen, R.; Caterino, T.D.; Terp, M.G.; Nissen, J.; Laenkholm, A.V.; Jensen, O.N.; Ditzel, H.J. NADH-Cytochrome b5 Reductase 3 Promotes Colonization and Metastasis Formation and Is a Prognostic Marker of Disease-Free and Overall Survival in Estrogen Receptor-Negative Breast Cancer. Mol. Cell. Proteom. 2015, 14, 2988–2999. [Google Scholar] [CrossRef]
- Wisniewska, A.; Jagiello, K.; Mazerska, Z. NADPH-cytochrome P450 reductase, not only the partner of cytochrome P450. Postepy Biochem. 2009, 55, 272–278. [Google Scholar]
- Rendic, S.P.; Crouch, R.D.; Guengerich, F.P. Roles of selected non-P450 human oxidoreductase enzymes in protective and toxic effects of chemicals: Review and compilation of reactions. Arch. Toxicol. 2022, 96, 2145–2246. [Google Scholar] [CrossRef] [PubMed]
- Zwierello, W.; Maruszewska, A.; Nowak, R.; Kostrzewa-Nowak, D.; Tarasiuk, J. DNA damage induced by NADPH cytochrome P450 reductase-activated idarubicin in sensitive and multidrug resistant MCF7 breast cancer cells. Pharmacol. Rep. 2017, 69, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, P.; Spiga, M.; Sani, M.; Zanda, M.; Fleming, I.N. KEMTUB012-NI2, a novel potent tubulysin analog that selectively targets hypoxic cancer cells and is potentiated by cytochrome p450 reductase downregulation. Hypoxia 2017, 5, 45–59. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Feng, Z.; Gao, S.; Wei, Y.; Han, S.; Wang, L. Contribution of NADPH-cytochrome P450 Reductase to Azole Resistance in Fusarium oxysporum. Front. Microbiol. 2021, 12, 709942. [Google Scholar] [CrossRef] [PubMed]
- Kuhbacher, A.; Merschak, P.; Haas, H.; Liebl, M.; Muller, C.; Gsaller, F. The cytochrome P450 reductase CprA is a rate-limiting factor for Cyp51A-mediated azole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2023, 67, e0091823. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Iqbal, J.; Krishan, A. Cytochrome P450 reductase, antioxidant enzymes and cellular resistance to doxorubicin. Biochem. Pharmacol. 1990, 40, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cytochrome P450 reductase (POR) as a ferroptosis fuel. Protein Cell 2021, 12, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Uruski, P.; Matuszkiewicz, K.; Szubert, S.; Moszynski, R.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Ksiazek, K. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. Cell. Oncol. 2016, 39, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Uruski, P.; Szubert, S.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Ksiazek, K. Malignant ascites determine the transmesothelial invasion of ovarian cancer cells. Int. J. Biochem. Cell Biol. 2017, 92, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Purbadi, S.; Anggraeni, T.D.; Vitria, A. Early stage epithelial ovarian cancer metastasis through peritoneal fluid circulation. J. Ovarian Res. 2021, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Pakula, M.; Mikula-Pietrasik, J.; Stryczynski, L.; Uruski, P.; Szubert, S.; Moszynski, R.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Ksiazek, K. Mitochondria-related oxidative stress contributes to ovarian cancer-promoting activity of mesothelial cells subjected to malignant ascites. Int. J. Biochem. Cell Biol. 2018, 98, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.; Kartikasari, A.E.R.; Plebanski, M. Elevated Interleukin-6 Levels in the Circulation and Peritoneal Fluid of Patients with Ovarian Cancer as a Potential Diagnostic Biomarker: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A. Lucigenin as a substrate of microsomal NAD(P)H-oxidoreductases. Biochemistry 1999, 64, 25–32. [Google Scholar] [PubMed]
- Baker, M.A.; Krutskikh, A.; Curry, B.J.; Hetherington, L.; Aitken, R.J. Identification of cytochrome-b5 reductase as the enzyme responsible for NADH-dependent lucigenin chemiluminescence in human spermatozoa. Biol. Reprod. 2005, 73, 334–342. [Google Scholar] [CrossRef]
- Baker, M.A.; Krutskikh, A.; Curry, B.J.; McLaughlin, E.A.; Aitken, R.J. Identification of cytochrome P450-reductase as the enzyme responsible for NADPH-dependent lucigenin and tetrazolium salt reduction in rat epididymal sperm preparations. Biol. Reprod. 2004, 71, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Rezende, F.; Prior, K.K.; Lowe, O.; Wittig, I.; Strecker, V.; Moll, F.; Helfinger, V.; Schnutgen, F.; Kurrle, N.; Wempe, F.; et al. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic. Biol. Med. 2017, 102, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Proskurnina, E.V.; Fedorova, M.V.; Sozarukova, M.M.; Mitichkin, A.E.; Panteleev, I.V.; Svetlov, E.V. Microsomal reductase activity in patients with thyroid neoplasms. Endocrine 2021, 72, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Proskurnina, E.V.; Fedorova, M.V.; Voznesensky, V.I.; Savinova, E.A. Activity of NAD(P)H-oxidoreductases and oxidative homeostasis in endometrial and cervical cancer. Tekhnologii Zhivykh Sist. 2023, 20, 31–44. (In Russian) [Google Scholar]
- Sozarukova, M.M.; Polimova, A.M.; Proskurnina, E.V.; Vladimirov, Y.A. Changes in the Kinetics of Plasma Chemiluminescence as a Measure of Systemic Oxidative Stress in Humans. Biophysics 2016, 61, 284–290. [Google Scholar] [CrossRef]
- Feng, J.; Liu, H.; Yang, X.; Gao, A.; Liao, J.; Feng, L.; Pu, J.; Xie, Y.; Long, G.; Li, Y.; et al. Comparison of activity indexes for recognizing enzyme mutants of higher activity with uricase as model. Chem. Cent. J. 2013, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.F.; Reinholz, N.; Leipert, B.; Raschke, P.; Permanetter, B.; Gerlach, E. Role of uric acid as an endogenous radical scavenger and antioxidant. Chest 1991, 100, 176S–181S. [Google Scholar] [CrossRef]
- Villalba, J.M.; Navarro, F.; Gomez-Diaz, C.; Arroyo, A.; Bello, R.I.; Navas, P. Role of cytochrome b5 reductase on the antioxidant function of coenzyme Q in the plasma membrane. Mol. Asp. Med. 1997, 18 (Suppl. S1), S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Mahmutoglu, I.; Kappus, H. Redox cycling of bleomycin-Fe(III) and DNA degradation by isolated NADH-cytochrome b5 reductase: Involvement of cytochrome b5. Mol. Pharmacol. 1988, 34, 578–583. [Google Scholar] [PubMed]
- Samhan-Arias, A.K.; Marques-da-Silva, D.; Yanamala, N.; Gutierrez-Merino, C. Stimulation and clustering of cytochrome b5 reductase in caveolin-rich lipid microdomains is an early event in oxidative stress-mediated apoptosis of cerebellar granule neurons. J. Proteom. 2012, 75, 2934–2949. [Google Scholar] [CrossRef] [PubMed]
- Bello, R.I.; Alcain, F.J.; Gomez-Diaz, C.; Lopez-Lluch, G.; Navas, P.; Villalba, J.M. Hydrogen peroxide- and cell-density-regulated expression of NADH-cytochrome b5 reductase in HeLa cells. J. Bioenerg. Biomembr. 2003, 35, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhao, W.; Tian, F.; Zhou, X.; Zhang, J.; Huang, T.; Hou, B.; Du, C.; Wang, S.; Mo, Y.; et al. Cytochrome b5 reductase 2 is a novel candidate tumor suppressor gene frequently inactivated by promoter hypermethylation in human nasopharyngeal carcinoma. Tumour Biol. 2014, 35, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.C.; Snyder, R.O.; Gumaste, U.; Thekkumkara, T.J.; Mehvar, R. Effects of transient overexpression or knockdown of cytochrome P450 reductase on reactive oxygen species generation and hypoxia reoxygenation injury in liver cells. Clin. Exp. Pharmacol. Physiol. 2011, 38, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Davydov, D.R.; Verma, S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Hrycay, E.G.; Bandiera, S.M. Involvement of Cytochrome P450 in Reactive Oxygen Species Formation and Cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar] [CrossRef] [PubMed]
- Proskurnina, E.V.; Fedorova, M.V.; Savinova, E.A.; Voznesenskii, V.I.; Kostyuk, S.V.; Sosnova, E.A. Oxidative Metabolism Genes in Ovarian Neoplasms. VF Snegirev Arch. Obstet. Gynecol. 2023, 10, 133–143. (In Russian) [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Taghizadeh, N.; Vonk, J.M.; Boezen, H.M. Serum uric acid levels and cancer mortality risk among males in a large general population-based cohort study. Cancer Causes Control 2014, 25, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.; Sookthai, D.; Graf, M.E.; Schubel, R.; Freisling, H.; Johnson, T.; Katzke, V.; Kaaks, R. Albumin, bilirubin, uric acid and cancer risk: Results from a prospective population-based study. Br. J. Cancer 2017, 117, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, H.R.; Lee, D.C.; Shim, J.Y.; Cho, K.H.; Suh, S.Y. Uric acid as a prognostic factor for survival time: A prospective cohort study of terminally ill cancer patients. J. Pain Symptom Manag. 2006, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, P.; Xu, W.; Liu, Y.; Wang, B.; Jiang, T.; Hua, C.; Wang, X.; Xu, D.; Sun, B. Serum Uric Acid Increases Risk of Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis. Mediat. Inflamm. 2015, 2015, 764250. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Yiu, A.; Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Malmstrom, H.; Lambe, M.; Hammar, N.; Walldius, G.; Jungner, I.; Wulaningsih, W. Circulating uric acid levels and subsequent development of cancer in 493,281 individuals: Findings from the AMORIS Study. Oncotarget 2017, 8, 42332–42342. [Google Scholar] [CrossRef] [PubMed]
- Stojnev, S.; Ristic-Petrovic, A.; Jankovic-Velickovic, L. Reactive oxygen species, apoptosis and cancer. Vojnosanit. Pregl. 2013, 70, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [PubMed]




| Index | Serous Cystadenoma (The Control Group) (n = 11), Mean (SD) | Well-Differentiated Adenocarcinoma (n = 15), Mean (SD) | Moderately Differentiated Adenocarcinoma (n = 13), Mean (SD) | Poorly Differentiated Adenocarcinoma (n = 14), Mean (SD) |
|---|---|---|---|---|
| I0 | 0.14 (0.08) | 0.20 (0.08) | 0.24 (0.18) | 0.95 * (0.45) |
| INADH | 0.88 (0.55) | 0.96 (0.25) | 7.28 * (3.55) | 8.73 * (3.67) |
| KNADH = (INADH − I0)/I0 | 6.08 (0.96) | 5.51 (0.90) | 25.83 * (9.41) | 21.50 * (10.11) |
| INADPH | 0.88 (0.62) | 1.71 (1.28) | 4.21 * (2.56) | 4.18 * (2.23) |
| KNADPH = (INADPH − I0)/I0 | 5.72 (1.25) | 8.15 (6.44) | 15.30 * (8.02) | 12.32 * (7.05) |
| Index | Non-Resistant Adenocarcinoma (n = 8), Mean (SD) | Chemoresistant Adenocarcinoma (n = 6), Mean (SD) |
|---|---|---|
| INADH | 8.48 (6.12) | 4.75 * (2.55) |
| INADPH | 7.65 (4.40) | 4.42 * (1.94) |
| Subgroup | Antioxidant Capacity, Mean (SD) |
|---|---|
| Serous cystadenoma (n = 11), the control group | 245 (130) |
| Well-differentiated adenocarcinoma (n = 15) | 422 * (231) |
| Moderately differentiated adenocarcinoma (n = 13) | 658 * (243) |
| Poorly differentiated adenocarcinoma (n = 14) | 579 * (260) |
| Index | INADH (Cells from Peritoneal Fluid) | INADPH (Cells from Peritoneal Fluid) |
|---|---|---|
| INADH (tissue) | 0.39 | — |
| INADPH (tissue) | — | 0.45 |
| Antioxidant capacity of peritoneal fluid for benign and highly differentiated tumors | 0.13 | 0.17 |
| Antioxidant capacity of peritoneal fluid for moderately and poorly differentiated tumors | 0.37 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorova, M.V.; Voznesensky, V.I.; Sosnova, E.A.; Proskurnina, E.V. Activity of NAD(P)H-Oxidoreductases in Ovarian Cancer. Biomedicines 2024, 12, 1052. https://doi.org/10.3390/biomedicines12051052
Fedorova MV, Voznesensky VI, Sosnova EA, Proskurnina EV. Activity of NAD(P)H-Oxidoreductases in Ovarian Cancer. Biomedicines. 2024; 12(5):1052. https://doi.org/10.3390/biomedicines12051052
Chicago/Turabian StyleFedorova, Maria V., Vladimir I. Voznesensky, Elena A. Sosnova, and Elena V. Proskurnina. 2024. "Activity of NAD(P)H-Oxidoreductases in Ovarian Cancer" Biomedicines 12, no. 5: 1052. https://doi.org/10.3390/biomedicines12051052





