Numerical Study of PBX 9501 Explosive Combustion Process in Confined Space
Abstract
:1. Introduction
2. Methodology
2.1. Combustion Rate Model
- Ignition stage of black powder. At this stage, the high-temperature gas produced by the combustion of black powder in the ignition chamber enters the crack, and the contact time between the surface of the explosive and the high-temperature combustion product produced by the combustion of black powder is short, so the combustion reaction of the explosive has not occurred.
- Phase of convection combustion and crack growth. The hot gas generated by the burning black powder propagates into the middle of the crack, initiating the combustion of a portion of the explosive near the ignition chamber. As the burning progresses, the explosive undergoes crack formation, allowing the high-temperature product gas to enter the crack, expanding the combustion surface area.
- The stage of rapid pressure rise. The continuous combustion of the upper and lower surface of the crack leads to increasing pressure inside the crack. When the pressure near the open end exceeds the pressure inside the ignition chamber, the product gas inside the crack flows into the ignition chamber through the open end. As the crack pressure continues to rise, the outlet’s flow velocity also increases.
- Restrain the shell breaking and pressure unloading stage. This stage is not within the research scope of this paper and will not be discussed in this paper.
2.2. Turbulence Model
2.3. Geometric Structure and Grid
2.4. Simulation Set-Ups
2.5. UDF Specification
3. Results and Discussion
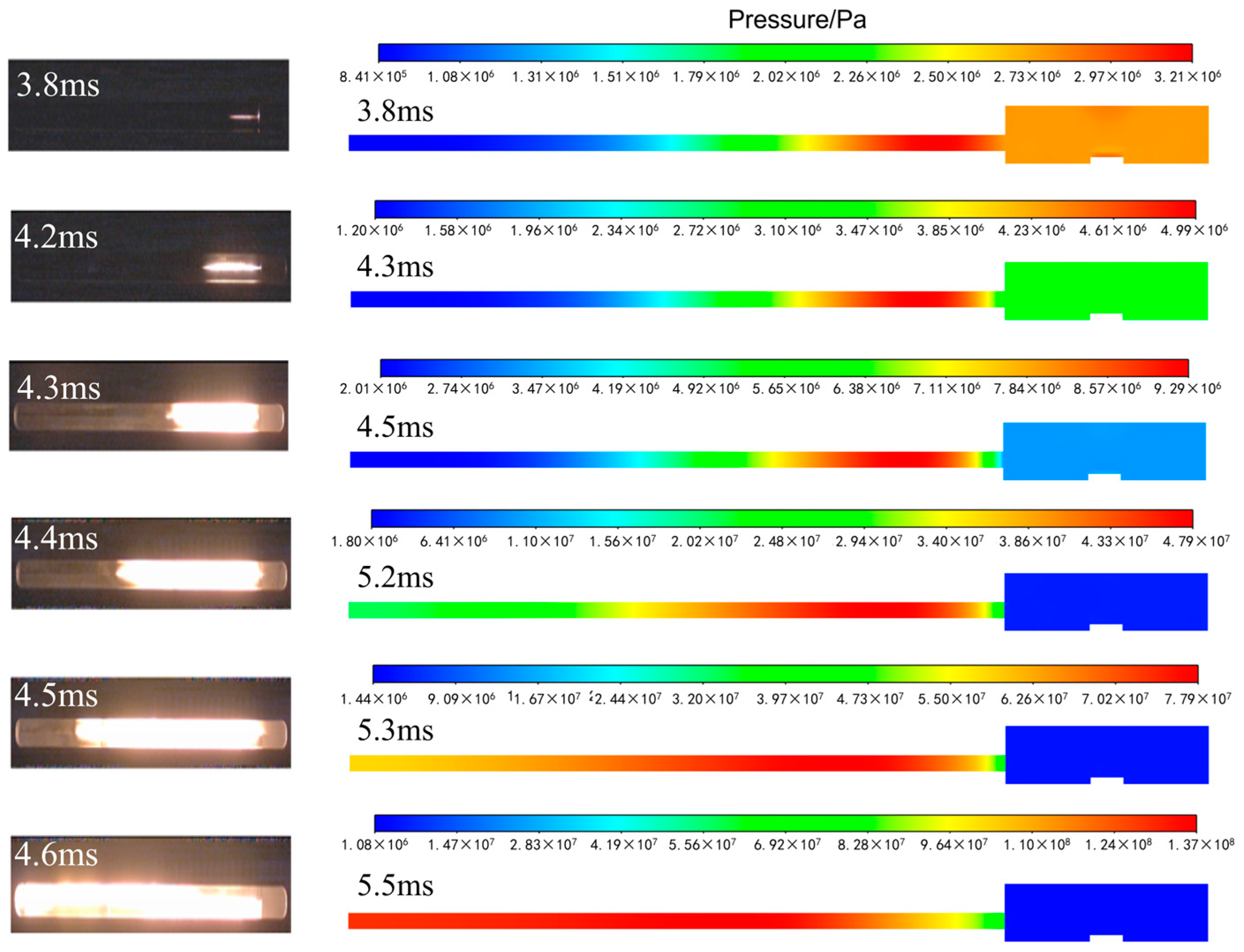
3.1. Comparative Analysis of Temperature Field
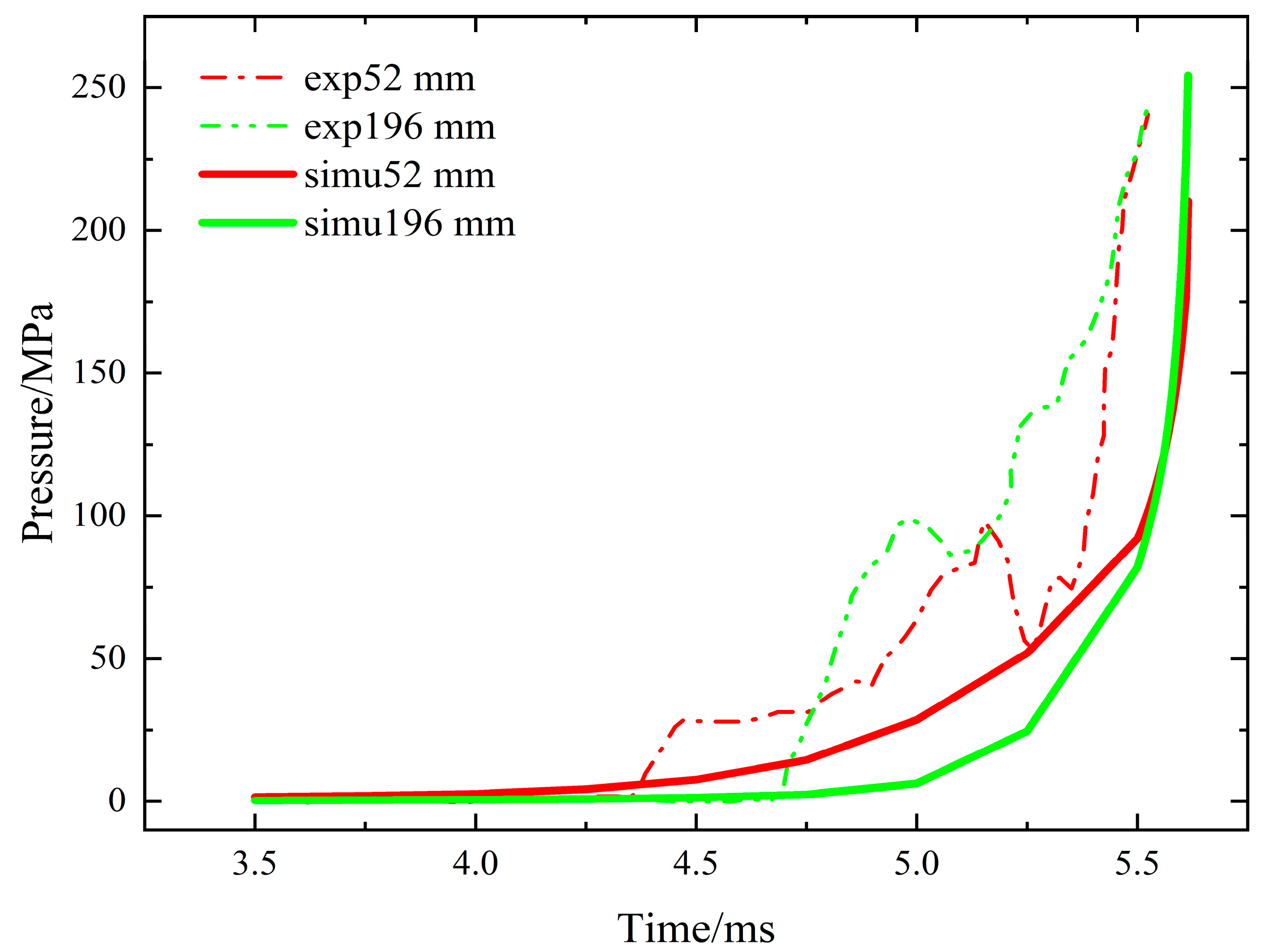
3.2. Comparative Analysis of Pressure Field
- The simulated pressure rise curve is basically consistent with that from the literature, showing an exponential rising trend.
- In the literature, when the explosive is burned to 5.5 ms, the pressure at each node rises to about 250 MPa. In the simulation, when the combustion reaches 5.6 ms, the pressure at 100 mm, 148 mm and 196 mm rises to about 250 MPa, the pressure at 52 mm rises to about 200 MPa and the pressure at 4 mm rises to about 100 MPa.
- In the experiment, the sequence of rising edges at nodes is 52 mm, 100 mm, 148 mm and 196 mm. In the simulation, the ascending edges at each node are in the same order of 52 mm, 100 mm, 148 mm and 196 mm. In addition, the time of each node’s rising edge in the experiment and simulation is distributed between 4.2 ms and 4.8 ms.
- In the experiment, the explosive may have uneven filling pressure and it is impossible to achieve a complete seal, resulting in more fluctuations and bending of the experimental curve. The simulation is an ideal explosive combustion in a completely closed container, so the curve shows a relatively smooth rise.
- In the experiment, the explosive itself may have local cracks, resulting in a partial pressure surge in the low-pressure section. In the simulation, the explosive is a uniform entity, so the curve shows a smooth rise.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBX | Plastic bonded explosive |
| MWSD | Modified Wescott–Stewart–Davis model |
| UDF | User-defined functions |
References
- Leuret, F.; Chaisse, F.; Presles, H.N. Experimental study of the low velocity detonation regime during the deflagration to detonation transition in a high density explosive. In Proceedings of the 11th International Detonation Symposium, Snowmass, CO, USA, 15–20 July 1998. [Google Scholar]
- Verbeek, R.; Steen, A.; Jontg, E. The influence of parameter variations on the deflagration to detonation transition. In Proceedings of the 10th International Detonation Symposium, Boston, MA, USA, 12–16 July 1993. [Google Scholar]
- Luebcke, P.E.; Dickson, P.M.; Field, J.E. Experimental investigation into the deflagration to detonation transition in secondary explosives. In Proceedings of the 10th International Detonation Symposium, Boston, MA, USA, 12–16 July 1993. [Google Scholar]
- Shang, H.; Yang, J.; Li, T. Experimental Study on Burning Evolution in Confined HMX-bsaed PBX Cracks. Chin. J. Energ. Mater. 2019, 27, 1056–1061. (In Chinese) [Google Scholar]
- Shang, H.; Yang, J.; Hu, Q. Experimental Research on Convective Burning in Explosive Cracks. Acta Armamentarii 2019, 40, 99–106. (In Chinese) [Google Scholar]
- Shang, H.; Hu, Q.; Li, T. One-dimensional theory for pressurization process in explosive crack burning. Explos. Shock Waves 2020, 40, 011403. (In Chinese) [Google Scholar]
- Hu, H.; Guo, Y.; Fu, H. The Dominant Mechanisms of Reaction Violence Transition in Explosive Accidents. Chin. J. Energ. Mater. 2016, 24, 622–624. (In Chinese) [Google Scholar]
- Asay, B.W.; Son, S.F.; Bdzil, J.B. The role of gas permeation in convective burning. Int. J. Multiph. Flow 1996, 22, 923–952. [Google Scholar] [CrossRef]
- Dickson, P.M.; Asay, B.W.; Henson, B.F. Thermal cook-off response of confined PBX 9501. Proc. Royal Soc. A 2004, 460, 3447–3455. [Google Scholar] [CrossRef]
- Dickson, P.M.; Asay, B.W.; Henson, B.F. Observation of the Behaviour of Confined PBX 9501. In Proceedings of the 11th International Detonation Symposium, Snowmass, CO, USA, 15–20 July 1998. [Google Scholar]
- Jackson, S.I.; Hill, L.G.; Berghout, H.L. Runaway reaction in a solid explosive containing a single crack. In Proceedings of the 13th International Detonation Symposium, Norfolk, VA, USA, 23–28 July 2006. [Google Scholar]
- Wescott, B.L.; Scott Stewart, D.; Davis, W.C. Equation of state and reaction rate for condensed-phase explosives. J. Appl. Phys. 2005, 98, 053514. [Google Scholar] [CrossRef]
- Berghout, H.; Steven, F.S.; Larry, G.H. Flame spread through cracks of PBX 9501 (a composite octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine-based explosive). J. Appl. Phys. 2006, 99, 114901. [Google Scholar] [CrossRef]
- Jackson, S.I.; Hill, L.G. Runaway reaction due to gas-dynamic choking in solid explosive containing a single crack. Proc. Combust. Inst. 2009, 32, 2307–2313. [Google Scholar] [CrossRef]
- Berghout, H.L.; Son, S.F.; Asay, B.W. Convective burning in gaps of PBX 9501. Proc. Combust. Inst. 2000, 28, 911–917. [Google Scholar] [CrossRef]
- Hang, S.; Yan, S.; Zhang, G. Computing Method of Environment Equivalent Temperature Based on Arrhenius Equation for Missile Products. Equip. Environ. Eng. 2018, 15, 74–77. (In Chinese) [Google Scholar]
- Short, M.; Anderson, E.K.; Chiquete, C. Experimental and modeling analysis of detonation in circular arcs of the conventional high explosive PBX 9501. Proc. Combust. Inst. 2021, 38, 3683–3690. [Google Scholar] [CrossRef]
- Chiquete, C.; Short, M.; Meyer, C.D. Calibration of the Pseudo-Reaction-Zone model for detonation wave propagation. Combust. Theory Model. 2018, 22, 744–776. [Google Scholar] [CrossRef]
- John, F.B.; Richard, D.D. Lasl Explosive Property Data; Terry, R.G., Charles, E.M., Stanley, P.M., Eds.; Los Alamos Data Center: Los Alamos, NM, USA, 1980; pp. 109–119. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Liu, J.; Wan, Q.; Zhang, M.; Li, M. Numerical Study of PBX 9501 Explosive Combustion Process in Confined Space. Processes 2023, 11, 2056. https://doi.org/10.3390/pr11072056
Hu Y, Liu J, Wan Q, Zhang M, Li M. Numerical Study of PBX 9501 Explosive Combustion Process in Confined Space. Processes. 2023; 11(7):2056. https://doi.org/10.3390/pr11072056
Chicago/Turabian StyleHu, Yupeng, Jiawen Liu, Qiang Wan, Meng Zhang, and Minghai Li. 2023. "Numerical Study of PBX 9501 Explosive Combustion Process in Confined Space" Processes 11, no. 7: 2056. https://doi.org/10.3390/pr11072056




