Study on the Influencing Factors of Injection Blockage during CO2 Sequestration in One-Dimensional Long Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Setup
2.3. Experimental Principle
2.4. Experimental Methods and Procedures
3. Results and Discussion
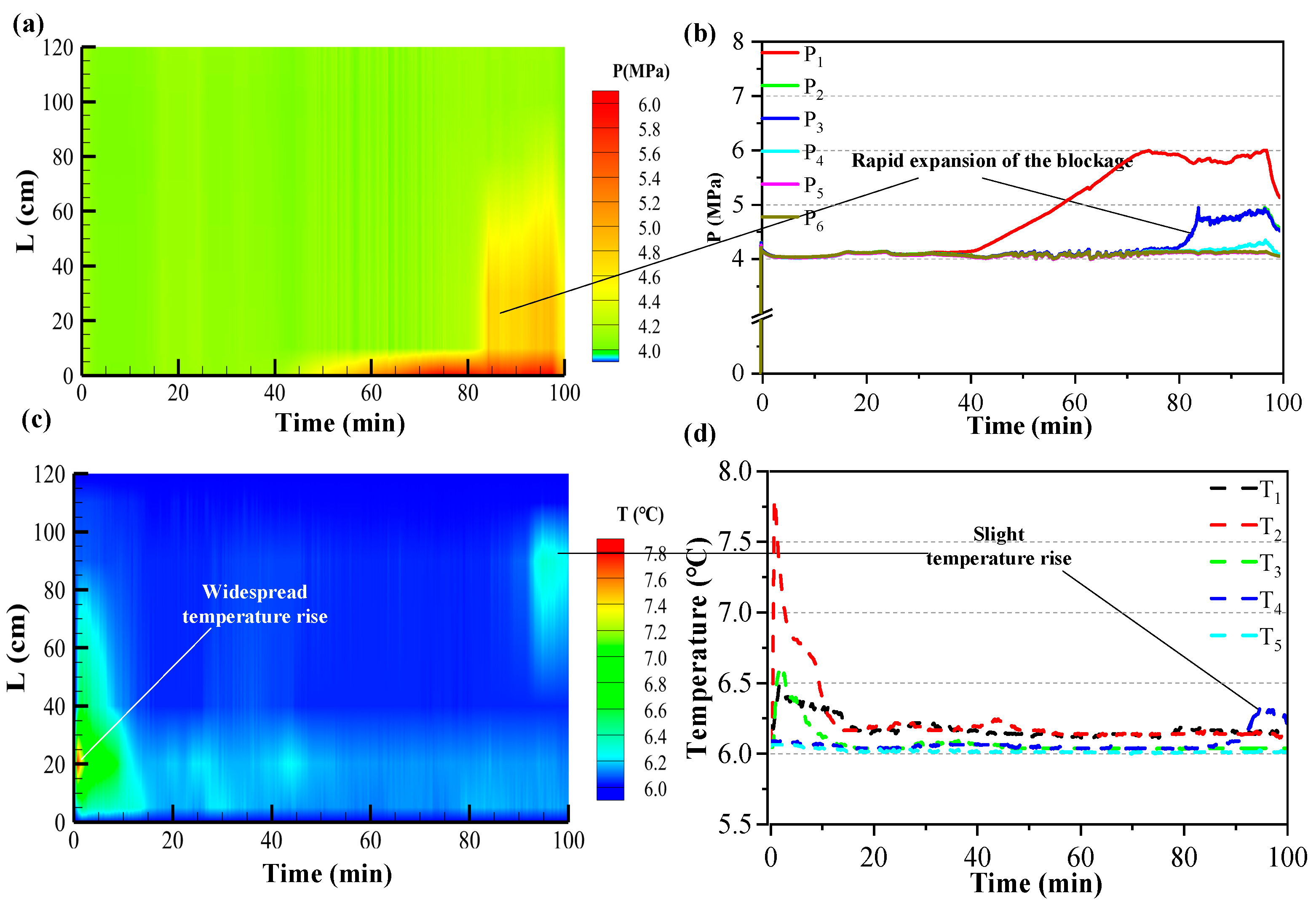
3.1. Effect of Sediment Temperature on CO2 Hydrate Formation
3.2. Effect of Different Initial Sediment Pressures on CO2 Hydrate Formation
3.3. Effect of Different Injection Rates on CO2 Hydrate Formation
4. Conclusions
- The study revealed that CO2 rapidly interacts with pore water to form significant amounts of hydrate, accompanied by heat release. This process not only increases the internal temperature of the reactor but also leads to a progressive increase in hydrate saturation. As hydrate forms, it extends the formation region, illustrating a dynamic system where the physical properties of the reactor environment are continuously evolving. It is of critical importance to note that the formation of dense hydrate structures within pore spaces can impede further CO2 injection by obstructing fluid flow channels. This finding serves to highlight the necessity of managing hydrate formation in order to optimize CO2 sequestration efficiency.
- The influence of pressure and temperature on hydrate formation has been the subject of considerable research. Our findings indicate that under conditions of low sediment pressure and high temperature, the driving force for CO2 hydrate formation is reduced, leading to slower hydrate formation rates. This slower formation rate mitigates the extent and severity of plugging, thereby enhancing the capacity for CO2 injection and sequestration. This observation is crucial for designing effective CO2 injection strategies that minimize operational disruptions caused by hydrate plugging.
- The impact of injection rate on system dynamics is a significant factor in the effectiveness of CO2 sequestration operations. Increasing the gas injection rate under constant temperature and pressure conditions has been shown to significantly reduce the contact time between CO2 gas and pore water, which alleviates hydrate plugging issues. This facilitates a broader distribution of gas injection, effectively increasing the volume of CO2 that can be sequestered. This finding suggests that optimizing injection rates can be a key strategy in maximizing the efficiency and effectiveness of CO2 sequestration operations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, Y.M.; Han, R.; Wang, C.; Yu, B.; Liang, Q.M.; Yuan, X.C.; Chang, J.; Zhao, Q.; Liao, H.; Tang, B. Self-preservation strategy for approaching global warming targets in the post-Paris Agreement era. Nat. Commun. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Gabitto, J.; Tsouris, C. Dissolution mechanisms of CO2 hydrate droplets in deep seawaters. Energy Convers. Manag. 2006, 47, 494–508. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Rehman, A.N.; Bavoh, C.B.; Pendyala, R.; Lal, B. Research Advances, Maturation, and Challenges of Hydrate-Based CO2 Sequestration in Porous Media. ACS Sustain. Chem. Eng. 2021, 9, 15075–15108. [Google Scholar] [CrossRef]
- Lim, D.; Ro, H.; Seo, Y.; Seo, Y.J.; Lee, J.Y.; Kim, S.J.; Lee, J.; Lee, H. Thermodynamic stability and guest distribution of CH4/N2/CO2 mixed hydrates for methane hydrate production using N2/CO2 injection. J. Chem. Thermodyn. 2017, 106, 16–21. [Google Scholar] [CrossRef]
- Yang, M.; Song, Y.; Jiang, L.; Zhao, Y.; Ruan, X.; Zhang, Y.; Wang, S. Hydrate-based technology for CO2 capture from fossil fuel power plants. Appl. Energy 2014, 116, 26–40. [Google Scholar] [CrossRef]
- Fahed Qureshi, M.; Zheng, J.; Khandelwal, H.; Venkataraman, P.; Usadi, A.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. Laboratory demonstration of the stability of CO2 hydrates in deep-oceanic sediments. Chem. Eng. J. 2022, 432, 134290. [Google Scholar] [CrossRef]
- Timur, A. Nuclear magnetic resonance study of carbonate rocks. Log Anal. 1972, 13, 3–11. [Google Scholar]
- Yang, M.; Song, Y.; Zhu, N.; Zhao, Y.; Liu, Y.; Jiang, L. Dynamic Measurements of CO2 Flow in Water Saturated Porous Medium at Low Temperature Using MRI. Energy Procedia 2013, 37, 1267–1274. [Google Scholar] [CrossRef]
- Yang, M.; Song, Y.; Jiang, L.; Zhu, N.; Liu, Y.; Zhao, Y.; Dou, B.; Li, Q. CO2 Hydrate Formation and Dissociation in Cooled Porous Media: A Potential Technology for CO2 Capture and Storage. Environ. Sci. Technol. 2013, 47, 9739–9746. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.n.; Yang, L.; Ma, S.; Zhao, Y.; Yang, M. Quantitative analysis of CO2 hydrate formation in porous media by proton NMR. AIChE J. 2020, 66, e16820. [Google Scholar] [CrossRef]
- Tian, H.; Wei, C.; Yan, R.; Chen, H. A NMR-based analysis of carbon dioxide hydrate dissociation process in silt. Sci. Sin. Phys. Mech. Astron. 2019, 49, 034615. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Shan, T.; Yuan, Q.; Yin, S.; Li, J.; Wu, Q.; Zhang, P. Molecular dynamics study of the influence of water molecular phase state on the replacement of CO2–CH4 hydrate in porous media. J. Mol. Liq. 2023, 391, 123401. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, S.; Li, S.; Wang, X.; Peng, S. Hydrate formation from clay bound water for CO2 storage. Chem. Eng. J. 2021, 406, 126872. [Google Scholar] [CrossRef]
- Pan, D.B.; Zhong, X.P.; Zhu, Y.; Zhai, L.H.; Zhang, H.; Li, X.T.; Wang, Y.F.; Chen, C. CH4 recovery and CO2 sequestration from hydrate-bearing clayey sediments via CO2/N2 injection. J. Nat. Gas Sci. Eng. 2020, 83, 103503. [Google Scholar] [CrossRef]
- Li, N.; Kan, J.-Y.; Sun, C.-Y.; Chen, G.-J. Hydrate formation from liquid CO2 in a glass beads bed. Chin. J. Chem. Eng. 2022, 43, 185–191. [Google Scholar] [CrossRef]
- Shindo, Y.; Lund, P.C.; Fujioka, Y.; Komiyama, H. Kinetics of formation of CO2 hydrate. Energy Convers. Manag. 1993, 34, 1073–1079. [Google Scholar] [CrossRef]
- Uchida, T.; Ebinuma, T.; Kawabata, J.i.; Narita, H. Microscopic observations of formation processes of clathrate-hydrate films at an interface between water and carbon dioxide. J. Cryst. Growth 1999, 204, 348–356. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, H.; Ling, Z.; Li, Y. Hydrate Formation Characteristics during Carbon Dioxide Flow Through Depleted Methane Hydrate Deposits. Energy Technol. 2018, 6, 1186–1195. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, G.; An, H.; Zhang, H. Generation laws and distribution characteristics of carbon dioxide hydrate in a reaction kettle. Exp. Therm. Fluid Sci. 2020, 116, 110125. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, H.; Ma, S.; Liu, W.; Yang, M. CO2 sequestration in depleted methane hydrate deposits with excess water. Int. J. Energy Res. 2018, 42, 2536–2547. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, J.W. Kinetic behaviors of CO2 hydrates in porous media and effect of kinetic promoter on the formation kinetics. Chem. Eng. Sci. 2010, 65, 1840–1845. [Google Scholar] [CrossRef]
- Li, B.; Sun, Y.; Jiang, S.; Shen, Y.; Qi, Y.; Zhang, G. Investigating CO2–N2 phase behavior for enhanced hydrate-based CO2 sequestration. Energy 2024, 289, 129946. [Google Scholar] [CrossRef]










| Case | Initial Temperature (°C) | Injection Rate (mL/min) | Initial Sediment Pressure (Mpa) | Piston Vessel Pressure (Mpa) | Injection Back-Pressure Valve Pressure (Mpa) |
|---|---|---|---|---|---|
| 1 | 4 | 10 | 3.7 | 4.2 | 4.2 |
| 2 | 6 | 10 | 3.7 | 4.2 | 4.2 |
| 3 | 8 | 10 | 3.7 | 4.2 | 4.2 |
| 4 | 4 | 20 | 2.7 | 3.2 | 3.2 |
| 5 | 4 | 20 | 3.2 | 3.7 | 3.7 |
| 6 | 4 | 20 | 3.7 | 4.2 | 4.2 |
| 7 | 6 | 20 | 3.7 | 4.2 | 4.2 |
| 8 | 6 | 30 | 3.7 | 4.2 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wei, H.; Liu, J.; Ma, X. Study on the Influencing Factors of Injection Blockage during CO2 Sequestration in One-Dimensional Long Reactor. Processes 2024, 12, 960. https://doi.org/10.3390/pr12050960
Zhang Y, Wei H, Liu J, Ma X. Study on the Influencing Factors of Injection Blockage during CO2 Sequestration in One-Dimensional Long Reactor. Processes. 2024; 12(5):960. https://doi.org/10.3390/pr12050960
Chicago/Turabian StyleZhang, Yi, Houzhen Wei, Jinxin Liu, and Xiaolong Ma. 2024. "Study on the Influencing Factors of Injection Blockage during CO2 Sequestration in One-Dimensional Long Reactor" Processes 12, no. 5: 960. https://doi.org/10.3390/pr12050960




