Effect of Obstacle Gradient on the Deflagration Characteristics of Hydrogen/Air Premixed Flame in a Closed Chamber
Abstract
:1. Introduction
2. Numerical Methods
2.1. Governing Equation
- The premixed gas is uniformly distributed before ignition.
- The wall is a non-slip and adiabatic boundary.
- The effects of gravity, thermal radiation, and heat loss are neglected owing to the reaction time being extremely short.
- The explosion gas is treated as a compressible ideal gas.
2.2. Combustion Model
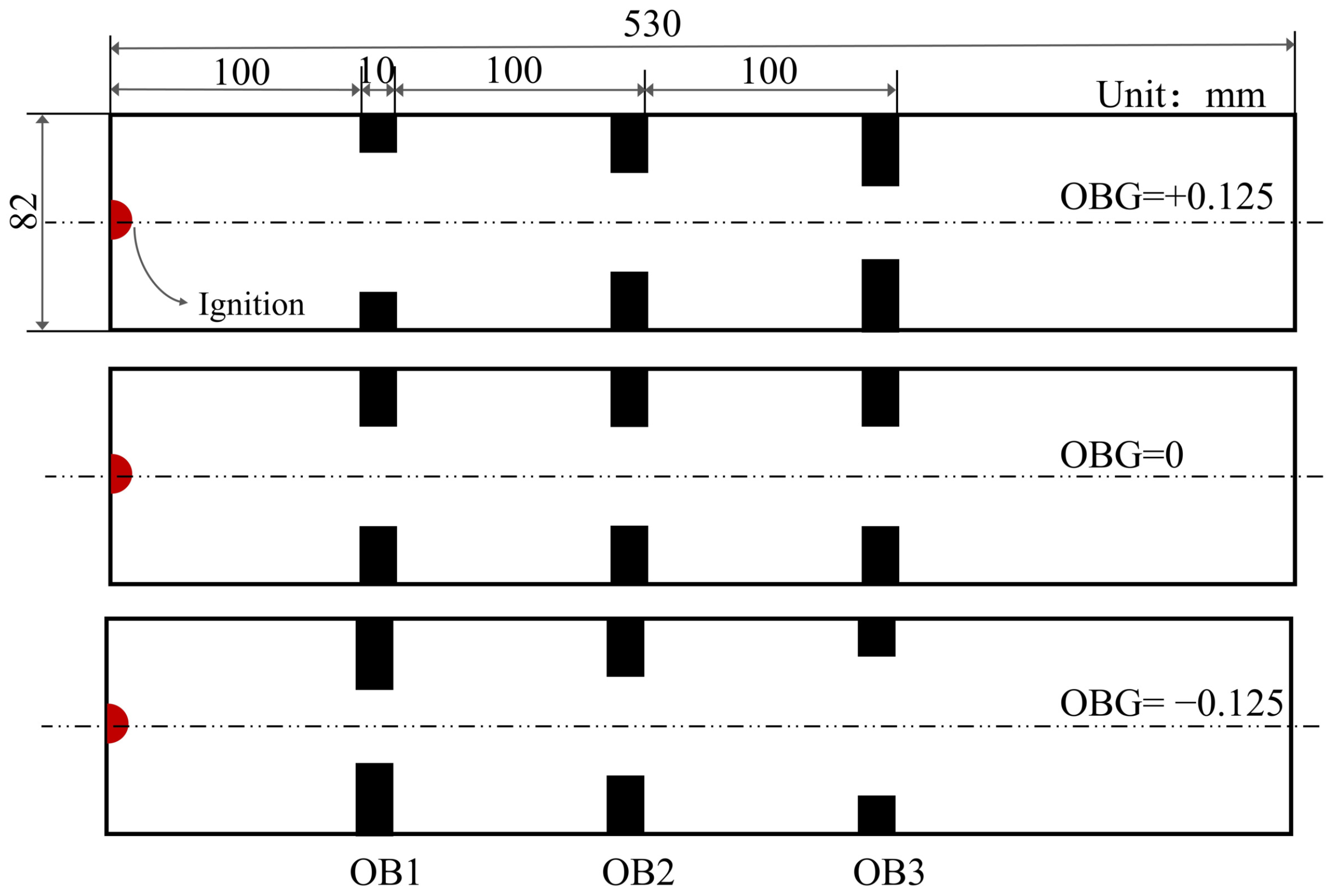
2.3. Geometry and Mesh
2.4. Initial Condition and Solver
2.5. Validation of Simulation Results
3. Results and Discussion
3.1. Analysis of Flame Propagation Structure
3.2. Kinetic Analysis of Flame Propagation Velocity
3.3. The Effect on Dynamic Overpressure
3.4. Flow Field under Different Obstacle Gradient Conditions
4. Conclusions
- The arrangement of the obstacle gradient can change the morphology of the explosion flame in the process. Overall, as the obstacle gradient increases, the flame front always maintains a jet-like shape, and the wrinkles on the flame front become more and more obvious.
- The blocking rate gradient has different effects on the flame propagation speed, which is enhanced by 8.1% (OBG = −0.125) and 19.8% (OBG = +0.125), respectively. The distribution of pressure is closely related to the changes in the flame structure, and the direction of the pressure gradient can also have different effects on the flame propagation speed. The blocking rate gradient will decrease the overpressure in obstacle channels to different degrees.
- An obvious vortex whose size is proportional to the blocking rate of the obstacle will be formed behind the obstacle during the flame propagation process. The vortex plays a key role during flame structure evolution, and more vortexes will be produced on the flame front with the increase in the obstacle gradient when passing channels.
- The arrangement of obstacles increases the gas flow velocity, and the larger the obstacle gradient, the greater the increase in flow velocity. But deceleration occurs at OBG = −0.125.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okere, C.J.; Sheng, J.J. Review on Clean Hydrogen Generation from Petroleum Reservoirs: Fundamentals, Mechanisms, and Field Applications. Int. J. Hydrogen Energy 2023, 48, 38188–38222. [Google Scholar] [CrossRef]
- Salehi, F.; Abbassi, R.; Asadnia, M.; Chan, B.; Chen, L. Overview of Safety Practices in Sustainable Hydrogen Economy—An Australian Perspective. Int. J. Hydrogen Energy 2022, 47, 34689–34703. [Google Scholar] [CrossRef]
- Norazahar, N.; Ambikabathy, T.M.; Kasmani, R.M.; Ahmad, A.; Jalil, A.A.; Abdullah, T.A.T.; Kamaroddin, M.F.A. Hydrogen Application and Its Safety: An Overview of Public Perceptions and Acceptance in Malaysia. Process Saf. Environ. Prot. 2023, 180, 686–698. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Z.; Yao, J.; Guo, T.; Yang, F.; Zhang, Z.; Ren, J.; Jiang, L.; Li, B. An Overview of Application-Oriented Multifunctional Large-Scale Stationary Battery and Hydrogen Hybrid Energy Storage System. Energy Rev. 2024, 3, 100068. [Google Scholar] [CrossRef]
- Li, J.C.; Xu, H.; Zhou, K.; Li, J.Q. A Review on the Research Progress and Application of Compressed Hydrogen in the Marine Hydrogen Fuel Cell Power System. Heliyon 2024, 10, e25304. [Google Scholar] [CrossRef]
- Goswami, R.; Sun, B. Study on Vapour Dispersion and Explosion from Compressed Hydrogen Spill: Risk Assessment on a Hydrogen Plant. Int. J. Hydrogen Energy 2022, 47, 41195–41207. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, Y.; Wang, S.; Pan, X.; Hua, M.; Wang, Z.; Wang, Q.; Li, Y.; Jiang, J. Numerical Study on the Flow Characteristics of Pressurized Hydrogen Leaking into the Confined Space through Different Shaped Orifices. Int. J. Hydrogen Energy 2022, 47, 35527–35539. [Google Scholar] [CrossRef]
- Guo, L.; Su, J.; Wang, Z.; Shi, J.; Guan, X.; Cao, W.; Ou, Z. Hydrogen Safety: An Obstacle That Must Be Overcome on the Road towards Future Hydrogen Economy. Int. J. Hydrogen Energy 2024, 51, 1055–1078. [Google Scholar] [CrossRef]
- Bivol, G.; Golovastov, S.; Golub, V. Effect of Channel Geometry and Porous Coverage on Flame Acceleration in Hydrogen–Air Mixture. Process Saf. Environ. Prot. 2021, 151, 128–140. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Wang, R.; Wang, H.; Wang, Q.; Liu, R.; Ma, H. Flame Behaviors and Overpressure Characteristics of the Unconfined Acetylene-Air Deflagration. Energy 2022, 246, 123380. [Google Scholar] [CrossRef]
- Cao, W.; Li, W.; Zhang, L.; Chen, J.; Yu, S.; Zhou, Z.; Zhang, Y.; Shen, X.; Tan, Y. Flame Characteristics of Premixed H2-air Mixtures Explosion Venting in a Spherical Container through a Duct. Int. J. Hydrogen Energy 2021, 46, 26693–26707. [Google Scholar] [CrossRef]
- Cao, W.; Zhou, Z.; Li, W.; Zhao, Y.; Yang, Z.; Zhang, Y.; Ouyang, S.M.; Shu, C.M.; Tan, Y. Under-Expansion Jet Flame Propagation Characteristics of Premixed H2/Air in Explosion Venting. Int. J. Hydrogen Energy 2021, 46, 38913–38922. [Google Scholar] [CrossRef]
- Shirvill, L.; Roberts, T.; Royle, M.; Willoughby, D.; Sathiah, P. Experimental Study of Hydrogen Explosion in Repeated Pipe Congestion—Part 2: Effects of Increase in Hydrogen Concentration in Hydrogen-Methane-Air Mixture. Int. J. Hydrogen Energy 2019, 44, 3264–3276. [Google Scholar] [CrossRef]
- Shen, R.; Jiao, Z.; Parker, T.; Sun, Y.; Wang, Q. Recent Application of Computational Fluid Dynamics (CFD) in Process Safety and Loss Prevention: A Review. J. Loss Prev. Process Ind. 2020, 67, 104252. [Google Scholar] [CrossRef]
- Sheng, Z.; Yang, G.; Li, S.; Shen, Q.; Sun, H.; Jiang, Z.; Liao, J.; Wang, H. Modeling of Turbulent Deflagration Behaviors of Premixed Hydrogen-Air in Closed Space with Obstacles. Process Saf. Environ. Prot. 2022, 161, 506–519. [Google Scholar] [CrossRef]
- Sun, X.; Lu, S. Effect of Obstacle Thickness on the Propagation Mechanisms of a Detonation Wave. Energy 2020, 198, 117186. [Google Scholar] [CrossRef]
- Boeck, L.; Lapointe, S.; Melguizo-Gavilanes, J.; Ciccarelli, G. Flame Propagation across an Obstacle: OH-PLIF and 2-D Simulations with Detailed Chemistry. Proc. Combust. Inst. 2017, 36, 2799–2806. [Google Scholar] [CrossRef]
- Yang, X.; Yu, M.; Zheng, K.; Luan, P.; Han, S. An Experimental Study on Premixed Syngas/Air Flame Propagating across an Obstacle in Closed Duct. Fuel 2020, 267, 117200. [Google Scholar] [CrossRef]
- Xiao, H.; Oran, E.S. Flame Acceleration and Deflagration-to-Detonation Transition in Hydrogen-Air Mixture in a Channel with an Array of Obstacles of Different Shapes. Combust. Flame 2020, 220, 378–393. [Google Scholar] [CrossRef]
- Oh, K.H.; Kim, H.; Kim, J.B.; Lee, S.E. A Study on the Obstacle-Induced Variation of the Gas Explosion Characteristics. J. Loss Prev. Process Ind. 2001, 14, 597–602. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, X. Flame Propagation of Premixed Hydrogen-Air Explosion in a Closed Duct with Obstacles. Int. J. Hydrogen Energy 2021, 46, 2684–2701. [Google Scholar] [CrossRef]
- Elshimy, M.; Ibrahim, S.; Malalasekera, W. Numerical Studies of Premixed Hydrogen/Air Flames in a Small-Scale Combustion Chamber with Varied Area Blockage Ratio. Int. J. Hydrogen Energy 2020, 45, 14979–14990. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, X. Study on the dynamic process of in-duct hydrogen-air explosion flame propagation under different blocking rates. Int. J. Hydrogen Energy 2022, 47, 18857–18876. [Google Scholar] [CrossRef]
- Mei, Y.; Shuai, J.; Li, Y.; Zhou, N.; Ren, W.; Ren, F. Flame Acceleration Process of Premixed Hydrogen in Confined Space with Different Obstacle Shapes. Fuel 2023, 334, 126624. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, W.; Sun, Z.; Liang, B.; Zhang, K.; Li, Y. Experimental and Numerical Study on Explosion Behavior of Hydrogen-Air Mixture in an Obstructed Closed Chamber. Int. J. Hydrogen Energy 2024, 49, 1032–1045. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, G.; Duan, Y.; Mi, H. Effect of Obstacle Arrangement on Premixed Hydrogen Flame: Eddy-dissipation Concept Model Based Numerical Simulation. Int. J. Hydrogen Energy 2023, 48, 16445–16456. [Google Scholar] [CrossRef]
- Xiu, Z.; Liu, Z.; Li, P.; Hao, B.; Li, M.; Zhao, Y.; Cai, P. Effects of Combined Obstacles on Deflagration Characteristics of Hydrogen-Air Premixed Gas. Int. J. Hydrogen Energy 2023, 79, 31008–31021. [Google Scholar] [CrossRef]
- Zheng, K.; Jia, Q.; Ma, Z.; Xing, Z.; Hao, Y.; Yu, M. Experimental and Numerical Investigation on the Premixed Methane/Air Flame Propagation in Duct with Obstacle Gradients. Process Saf. Environ. Prot. 2023, 178, 893–904. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, G.; Feng, Y.; Mi, H. Investigation of Premixed Hydrogen/Methane Flame Propagation and Kinetic Characteristics for Continuous Obstacles with Gradient Barrier Ratio. Energy 2023, 267, 126620. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Mulenga, L. Impact of Radiative Losses on Flame Acceleration and Deflagration to Detonation Transition of Lean Hydrogen-Air Mixtures in a Macro-Channel with Obstacles. Fluids 2018, 3, 104. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Xiao, H. Flame Acceleration and Deflagration-to-Detonation Transition in Narrow Channels Filled with Stoichiometric Hydrogen-Air Mixture. Int. J. Hydrogen Energy 2022, 47, 11052–11067. [Google Scholar] [CrossRef]
- Shen, T.; Li, M.; Xiao, H. Propagation of Premixed Hydrogen-Air Flame Initiated by a Planar Ignition in a Closed Tube. Int. J. Hydrogen Energy 2022, 47, 4903–4915. [Google Scholar] [CrossRef]
- Yakhot, V.; Orszag, S.A. Renormalization Group Analysis of Turbulence. I. Basic Theory. J. Sci. Comput. 1986, 1, 3–51. [Google Scholar] [CrossRef]
- Magnussen, B.F.; Hjertager, B.H. On Mathematical Modeling of Turbulent Combustion with Special Emphasis on Soot Formation and Combustion. Symp. Int. Combust. 1977, 16, 719–729. [Google Scholar] [CrossRef]
- Mansourian, M.; Kamali, R. Modifying the Constant Coefficients of Eddy-dissipation Concept Model in Moderate or Intense Low-Oxygen Dilution Combustion Using Inverse Problem Methodology. Acta Astronaut. 2019, 162, 546–554. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Kazakov, A.; Dryer, F.L. An Updated Comprehensive Kinetic Model of Hydrogen Combustion. Int. J. Chem. Kinet. 2004, 36, 566–575. [Google Scholar] [CrossRef]
- Zhou, G.; Kong, Y.; Qian, X.; Zhang, Q.; Ma, Y.; Wu, D. Explosion Dynamics and Sensitivity Analysis of Blended LPG/DME Clean Fuel Promoted by H2 in a Confined Elongated Space. Fuel 2023, 331, 125816. [Google Scholar] [CrossRef]
- Khodadadi Azadboni, R.; Heidari, A.; Boeck, L.R.; Wen, J.X. The Effect of Concentration Gradients on Deflagration-to-Detonation Transition in a Rectangular Channel with and without Obstructions—A Numerical Study. Int. J. Hydrogen Energy 2019, 44, 7032–7040. [Google Scholar] [CrossRef]
- Nguyen, T.; Strebinger, C.; Bogin, G.; Brune, J. A 2D CFD Model Investigation of the Impact of Obstacles and Turbulence Model on Methane Flame Propagation. Process Saf. Environ. Prot. 2021, 146, 95–107. [Google Scholar] [CrossRef]
- Xiao, H.; Sun, J.; Chen, P. Experimental and Numerical Study of Premixed Hydrogen/Air Flame Propagating in a Combustion Chamber. J. Hazard. Mater. 2014, 268, 132–139. [Google Scholar] [CrossRef]
- Xiao, H.; An, W.; Duan, Q.; Sun, J. Dynamics of Premixed Hydrogen/Air Flame in a Closed Combustion Vessel. Int. J. Hydrogen Energy 2013, 38, 12856–12864. [Google Scholar] [CrossRef]
- Xiao, H.; Makarov, D.; Sun, J.; Molkov, V. Experimental and Numerical Investigation of Premixed Flame Propagation with Distorted Tulip Shape in a Closed Duct. Combust. Flame 2012, 159, 1523–1538. [Google Scholar] [CrossRef]
- Clanet, C.; Searby, G. On the “tulip flame” phenomenon. Combust. Flame 1996, 105, 225–238. [Google Scholar] [CrossRef]
- Sheng, Z.; Yang, G.; Gao, W.; Li, S.; Shen, Q.; Sun, H. Study on the Dynamic Process of Premixed Hydrogen-Air Deflagration Flame Propagating in a Closed Space with Obstacles. Fuel 2023, 334, 126542. [Google Scholar] [CrossRef]











| No. | Process | A | n | E |
|---|---|---|---|---|
| 1 | H + O2 ⇒ O + OH | 3.55 × 1015 | −0.41 | 16.60 |
| 2 | O + H2 ⇒ H + OH | 5.08 × 104 | 2.67 | 6.29 |
| 3 | H2 + OH ⇒ H2O + H | 2.16 × 108 | 1.51 | 3.43 |
| 4 | O + H2O ⇒ OH + OH | 2.97 × 106 | 2.02 | 13.40 |
| 5 | H2 + N2 ⇒ H + H + N2 | 4.58 × 1019 | −1.40 | 104.38 |
| 6 | O + O + N2 ⇒ O2 + N2 | 6.16 × 1015 | -0.50 | 0.00 |
| 7 | O + H + N2 ⇒ OH + N2 | 4.71 × 1018 | −1.00 | 0.00 |
| 8 | H + OH + N2 ⇒ H2O + N2 | 3.80 × 1022 | −2.00 | 0.00 |
| 9 | H + O2 + N2 ↔ HO2 + N2 | 6.37 × 1020 | −1.72 | 0.52 |
| 10 | HO2 + H ⇒ H2 + O2 | 1.66 × 1013 | 0.00 | 0.82 |
| 11 | HO2 + H ⇒ OH + OH | 7.08 × 1013 | 0.00 | 0.30 |
| 12 | HO2 + O ⇒ OH + O2 | 3.25 × 1013 | 0.00 | 0.00 |
| 13 | HO2 + OH ⇒ H2O + O2 | 2.89 × 1013 | 0.00 | −0.50 |
| 14 | HO2 + HO2 ⇒ H2O2 + O2 | 4.20 × 1014 | 0.00 | 11.98 |
| 15 | H2O2 + N2 ↔ OH + OH + N2 | 1.20 × 1017 | 0.00 | 45.50 |
| 16 | H2O2 + H ⇒ H2O + OH | 2.41 × 1013 | 0.00 | 3.97 |
| 17 | H2O2 + H ⇒ H2 + HO2 | 4.82 × 1013 | 0.00 | 7.95 |
| 18 | H2O2 + O ⇒ OH + HO2 | 9.55 × 106 | 2.00 | 3.97 |
| 19 | H2O2 + OH ⇒ H2O + HO2 | 1.00 × 1012 | 0.00 | 0.00 |
| Obstacle Blocking Rate Gradient (OBG) | ||||
|---|---|---|---|---|
| Configuration | BR1 | BR2 | BR3 | Variable |
| Case1 | 0.375 | 0.5 | 0.625 | OBG = +0.125 |
| Case2 | 0.5 | 0.5 | 0.5 | OBG = 0 |
| Case3 | 0.625 | 0.5 | 0.375 | OBG = −0.125 |
| Flame Stage | Time Type (ms) | Empirical | Experimental [41] | Numerical |
|---|---|---|---|---|
| Spherical flame | tsphere | 1.95 ± 0.39 | 2.3 | 2 |
| Finger flame | twall | 5.08 ± 0.39 | 4.3 | 4 |
| Tulip flame | ttulip | 6.44 ± 0.39 | 5.6 | 5.6 |
| Configuration | Obstcle1 (m/s) | Obstcle2 (m/s) | Obstcle3 (m/s) | Increase in Flow Velocity |
|---|---|---|---|---|
| Case1 | 243 | 542 | 1122 | 123% and 107% |
| Case2 | 433 | 712 | 922 | 64% and 29% |
| Case3 | 670 | 759 | 733 | 12% and −3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhong, S. Effect of Obstacle Gradient on the Deflagration Characteristics of Hydrogen/Air Premixed Flame in a Closed Chamber. Processes 2024, 12, 962. https://doi.org/10.3390/pr12050962
Wang Y, Zhong S. Effect of Obstacle Gradient on the Deflagration Characteristics of Hydrogen/Air Premixed Flame in a Closed Chamber. Processes. 2024; 12(5):962. https://doi.org/10.3390/pr12050962
Chicago/Turabian StyleWang, Yufei, and Shengjun Zhong. 2024. "Effect of Obstacle Gradient on the Deflagration Characteristics of Hydrogen/Air Premixed Flame in a Closed Chamber" Processes 12, no. 5: 962. https://doi.org/10.3390/pr12050962




