Effect of Fiber and Insect Powder Addition on Selected Organoleptic and Nutritional Characteristics of Gluten-Free Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Breads
2.3. Experimental Design
2.4. Sensory Analysis
2.5. Texture Analysis
2.6. Visual Inspection by Optical Microscopy
2.7. Determination of Total Polyphenol Content and Antioxidant Capacity
2.7.1. Extraction of Bioactive Substances
2.7.2. Total Polyphenol Content (TPC)
2.7.3. ABTS Assay
2.7.4. DPPH Assay
2.7.5. Determination of Glycemic Index
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fiber Addition on Key Organoleptic Properties of Experimental Samples of Gluten-Free Bread Samples
Quantitative Descriptive Analysis
3.2. Hedonic Analysis
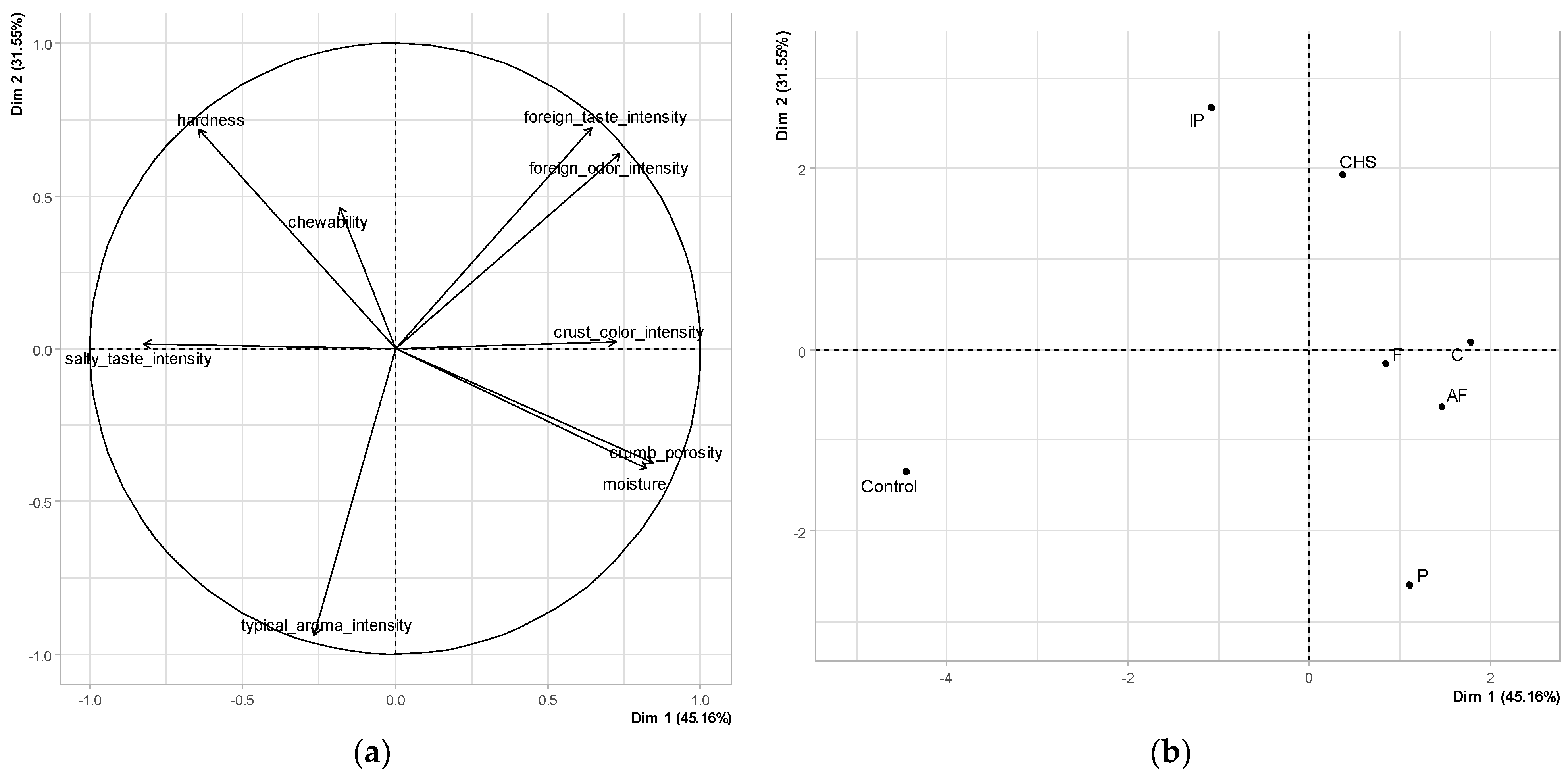
3.3. Effect of Fiber Type on Key Organoleptic Properties of Groups of Gluten-Free Bread Samples
3.3.1. Quantitative Descriptive Analysis
3.3.2. Hedonic Analysis
3.4. Instrumental Texture Determination
3.5. Visual Inspection by Optical Microscopy
3.6. Selected Nutritional Characteristics
3.6.1. Content of Polyphenols and Antioxidant Capacity
3.6.2. Glycemic Index
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Puerta, P.; Laguna, L.; Villegas, B.; Rizo, A.; Fiszman, S.; Tarrega, A. Oral processing and dynamics of texture perception in commercial gluten-free breads. Food Res. Int. 2020, 134, 109233. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Angadi, S.S. Gluten-free diet: Imprudent dietary advice for the general population? J. Acad. Nutr. Diet. 2012, 112, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Shah, M.A.; Hamdani, A.M. Gluten-Free Bread Technology; Springer: Heidelberg, Germany, 2021; ISBN 9783030738983. [Google Scholar]
- Scherf, K.A. Immunoreactive cereal proteins in wheat allergy, non-celiac gluten/wheat sensitivity (NCGS) and celiac disease. Curr. Opin. Food Sci. 2019, 25, 35–41. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Mendoza, M.C.; Beecroft, C.A.; Kerr, A.C.; Stathopoulos, C.E.; Fiore, A. Development of a gluten free bread enriched with faba bean husk as a fibre supplement. LWT 2023, 173, 114362. [Google Scholar] [CrossRef]
- Saboo, B.; Misra, A.; Kalra, S.; Mohan, V.; Aravind, S.R.; Joshi, S.; Chowdhury, S.; Sahay, R.; Kesavadev, J.; John, M.; et al. Role and importance of high fiber in diabetes management in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102480. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.M.R.; Guiné, R.P.F.; Fonseca, M.; Batista, L. Analysis of textural properties of gluten free breads. J. Hyg. Eng. Des. 2021, 34, 102–108. [Google Scholar]
- Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Djordjević, M.; Šereš, Z.; Milašinović-Šeremešić, M. Sugar beet and apple fibres coupled with hydroxypropylmethylcellulose as functional ingredients in gluten-free formulations: Rheological, technological and sensory aspects. Food Chem. 2019, 295, 189–197. [Google Scholar] [CrossRef]
- Mazzeo, T.; Brambillasca, F.; Pellegrini, N.; Valmarana, R.; Corti, F.; Colombo, C.; Agostoni, C. Evaluation of visual and taste preferences of some gluten-free commercial products in a group of celiac children. Int. J. Food Sci. Nutr. 2014, 65, 112–116. [Google Scholar] [CrossRef]
- de Kock, H.L.; Magano, N.N. Sensory tools for the development of gluten-free bakery foods. J. Cereal Sci. 2020, 94, 102990. [Google Scholar] [CrossRef]
- Ozyigit, E.; Eren, İ.; Kumcuoglu, S.; Tavman, S. Large Amplitude Oscillatory Shear (LAOS) analysis of gluten-free cake batters: The effect of dietary fiber enrichment. J. Food Eng. 2020, 275, 109867. [Google Scholar] [CrossRef]
- Sabanis, D.; Lebesi, D.; Tzia, C. Development of fibre-enriched gluten-free bread: A response surface methodology study. Int. J. Food Sci. Nutr. 2009, 60, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; De Jesus, J.M.; Desilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020-2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- van Megen, F.; Fossli, M.; Skodje, G.I.; Carlsen, M.H.; Andersen, L.F.; Veierød, M.B.; Lundin, K.E.A.; Henriksen, C. Nutritional assessment of women with celiac disease compared to the general population. Clin. Nutr. ESPEN 2023, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Larretxi, I.; Churruca, I.; Navarro, V.; Miranda, J.; Lasa, A.; Bustamante, M.Á.; Simon, E. Effect of analytically measured fiber and resistant starch from gluten-free products on the diets of individuals with celiac disease. Nutrition 2020, 70, 110586. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Al-Foudari, M.; Sidhu, J.S.; Alhazza, A. Effect of psyllium husk and wheat mill bran fractions on the microstructure and mixograph characteristics of Arabic bread. Saudi J. Biol. Sci. 2022, 29, 103479. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.M.; da Costa, D.V.; Trombete, F.M.; Câmara, A.K.F.I. Edible insects as a sustainable alternative to food products: An insight into quality aspects of reformulated bakery and meat products. Curr. Opin. Food Sci. 2022, 46, 100864. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat bread supplementation with various edible insect flours. Influence of chemical composition on nutritional and technological aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- van Huis, A.; Rumpold, B. Strategies to convince consumers to eat insects? A review. Food Qual. Prefer. 2023, 110, 104927. [Google Scholar] [CrossRef]
- Talens, C.; Lago, M.; Simó-Boyle, L.; Odriozola-Serrano, I.; Ibargüen, M. Desirability-based optimization of bakery products containing pea, hemp and insect flours using mixture design methodology. LWT 2022, 168, 113878. [Google Scholar] [CrossRef]
- Mishyna, M.; Chen, J.; Benjamin, O. Sensory attributes of edible insects and insect-based foods—Future outlooks for enhancing consumer appeal. Trends Food Sci. Technol. 2020, 95, 141–148. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and Volatilome characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Gumienna, M.; Rybicka, I.; Górna, B.; Sarbak, P.; Dziedzic, K.; Kmiecik, D. Nutritional value and biological activity of gluten-free bread enriched with cricket powder. Molecules 2021, 26, 1184. [Google Scholar] [CrossRef]
- Pao, S.; Kim, C.; Jordan, L.; Long, W.; Inserra, P.; Sayre, B. Growth of salmonella enterica and staphylococcus aureus in no-knead bread dough during prolonged yeast fermentation. J. Food Prot. 2011, 74, 285–288. [Google Scholar] [CrossRef]
- Tauferova, A.; Pospiech, M.; Javurkova, Z.; Tremlova, B.; Dordevic, D.; Jancikova, S.; Tesikova, K.; Zdarsky, M.; Vitez, T.; Vitezova, M. Plant byproducts as part of edible coatings: A case study with parsley, grape and blueberry pomace. Polymers 2021, 13, 2578. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Pankiewicz, U.; Sujka, M. Nutritional, physiochemical, and biological value of muffins enriched with edible insects flour. Antioxidants 2021, 10, 1122. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U. Nutritional, physiochemical, and antioxidative characteristics of shortcake biscuits enriched with Tenebrio molitor flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Filipčev, B.; Pojić, M.; Šimurina, O.; Mišan, A.; Mandić, A. Psyllium as an improver in gluten-free breads: Effect on volume, crumb texture, moisture binding and staling kinetics. LWT 2021, 151, 112156. [Google Scholar] [CrossRef]
- Pecova, M.; Tauferova, A.; Pospiech, M.; Bartlova, M.; Tremlova, B. Evaluation of Gluten-Free Bars Made with House Cricket (Acheta domesticus) Powder. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e9873. [Google Scholar] [CrossRef]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Aldughpassi, A.; Alkandari, S.; Alkandari, D.; Al-Hassawi, F.; Sidhu, J.S.; Al-Amiri, H.A.; Al-Salem, E. Effect of psyllium fiber addition on the quality of Arabic flatbread (Pita) produced in a commercial bakery. Ann. Agric. Sci. 2021, 66, 115–120. [Google Scholar] [CrossRef]
- Grancieri, M.; Verediano, T.A.; Sant’Ana, C.T.; de Assis, A.; Toledo, R.L.; de Mejia, E.G.; Martino, H.S.D. Digested protein from chia seed (Salvia hispanica L.) prevents obesity and associated inflammation of adipose tissue in mice fed a high-fat diet. PharmaNutrition 2022, 21, 100298. [Google Scholar] [CrossRef]
- Ahlborn, G.J.; Pike, O.A.; Hendrix, S.B.; Hess, W.M.; Huber, C.S. Sensory, mechanical, and microscopic evaluation of staling in low-protein and gluten-free breads. Cereal Chem. 2005, 82, 328–335. [Google Scholar] [CrossRef]
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Curti, E.; Carini, E.; Tribuzio, G.; Vittadini, E. Effect of bran on bread staling: Physico-chemical characterization and molecular mobility. J. Cereal Sci. 2015, 65, 25–30. [Google Scholar] [CrossRef]
- Zhu, F.; Chan, C. Effect of chia seed on glycemic response, texture, and sensory properties of Chinese steamed bread. LWT 2018, 98, 77–84. [Google Scholar] [CrossRef]
- García-Salcedo, Á.J.; Torres-Vargas, O.L.; del Real, A.; Contreras-Jiménez, B.; Rodriguez-Garcia, M.E. Pasting, viscoelastic, and physicochemical properties of chia (Salvia hispanica L.) flour and mucilage. Food Struct. 2018, 16, 59–66. [Google Scholar] [CrossRef]
- Torres, M.D.; Arufe, S.; Chenlo, F.; Moreira, R. Coeliacs cannot live by gluten-free bread alone—Every once in awhile they need antioxidants. Int. J. Food Sci. Technol. 2017, 52, 81–90. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Soengas, P.; Landa, A.; Ordás, A.; Revilla, P. Effects of selection for color intensity on antioxidant capacity in maize (Zea mays L.). Euphytica 2013, 193, 339–345. [Google Scholar] [CrossRef]
- Sakač, M.; Pestorić, M.; Mišan, A.; Nedeljković, N.; Jambrec, D.; Jovanov, P.; Banjac, V.; Torbica, A.; Hadnadev, M.; Mandić, A. Antioxidant capacity, mineral content and sensory properties of gluten-free rice and buckwheat cookies. Food Technol. Biotechnol. 2015, 53, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Tilley, M.; Li, Y. Changes in phenolic profiles and antioxidant activities during the whole wheat bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef] [PubMed]
- Abdel-aal, E.S.M.; Rabalski, I. Changes in Phenolic Acids and Antioxidant Properties during Baking of Bread and Muffin Made from Blends of Hairless Canary Seed, Wheat, and Corn. Antioxidants 2022, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Tontul, S.; Candal Uslu, C.; Mutlu, C.; Erbaş, M. Expected glycemic impact and probiotic stimulating effects of whole grain flours of buckwheat, quinoa, amaranth and chia. J. Food Sci. Technol. 2022, 59, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. In vitro starch digestibility and predicted glycaemic indexes of buckwheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. J. Cereal Sci. 2013, 58, 431–436. [Google Scholar] [CrossRef]
- Kurek, M.A.; Wyrwisz, J.; Karp, S.; Wierzbicka, A. Effect of fiber sources on fatty acids profile, glycemic index, and phenolic compound content of in vitro digested fortified wheat bread. J. Food Sci. Technol. 2018, 55, 1632–1640. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]






| Fiber Addition/Type | 2% | 5% | 7% | 9% | 13% | 17% | 23% |
|---|---|---|---|---|---|---|---|
| Flaxseed | – | – | – | F_9 | F_13 | F_17 | F_23 |
| Psyllium | P_2 | P_5 | P_7 | P_9 | – | – | – |
| Apple fiber | A_2 | A_5 | A_7 | A_9 | – | – | – |
| Chia seed | CH_2 | CH_5 | – | CH_9 | CH_13 | – | – |
| Insect powder | I_2 | I_5 | – | I_9 | I_13 | – | – |
| Sample/Fiber Type | C_1 | C_2 | C_3 | C_4 |
|---|---|---|---|---|
| Flaxseed | 5% | 4% | 8% | 8% |
| Psyllium | 2% | 2% | 2% | 2% |
| Apple fiber | 2% | 2% | 4% | 4% |
| Chia seed | 2% | 2% | 2% | 2% |
| Insect powder | – | 2% | – | 2% |
| Aroma | Appearance | Texture | Taste | Overall Evaluation | |
|---|---|---|---|---|---|
| P_9 | 5.607 | 3.334 | 3.610 | 4.297 | 3.885 |
| I_13 | 4.180 | 5.949 | 5.306 | 4.524 | 4.973 |
| I_5 | 5.487 | 5.718 | 4.845 | 4.678 | 4.665 |
| A_9 | 5.684 | 6.069 | 5.522 | 5.004 | 5.072 |
| F_23 | 5.981 | 4.910 | 5.037 | 5.879 | 5.141 |
| P_7 | 6.376 | 5.026 | 5.148 | 5.527 | 5.500 |
| CH_9 | 5.859 | 4.475 | 5.123 | 6.315 | 5.717 |
| CH_13 | 6.288 | 5.046 | 5.695 | 5.744 | 6.288 |
| CH_2 | 6.002 | 7.046 | 4.980 | 6.315 | 5.717 |
| CH_5 | 5.716 | 5.332 | 6.409 | 6.030 | 6.574 |
| I_2 | 6.564 | 5.949 | 5.845 | 5.678 | 6.126 |
| C_2 | 5.794 | 6.341 | 5.505 | 6.116 | 6.536 |
| Ctrl | 6.436 | 5.654 | 5.743 | 6.209 | 6.258 |
| A_2 | 6.041 | 6.283 | 6.379 | 5.718 | 6.144 |
| F_17 | 6.203 | 5.798 | 6.259 | 6.435 | 5.919 |
| C_4 | 5.885 | 6.978 | 5.414 | 6.116 | 6.536 |
| A_7 | 6.113 | 6.998 | 5.950 | 6.075 | 5.715 |
| F_13 | 6.648 | 6.132 | 6.481 | 6.213 | 5.808 |
| P_5 | 6.068 | 6.103 | 6.456 | 5.989 | 5.500 |
| I_9 | 5.795 | 7.026 | 6.922 | 5.909 | 6.434 |
| A_5 | 5.827 | 6.783 | 6.236 | 6.504 | 6.501 |
| C_1 | 5.883 | 6.426 | 6.112 | 6.511 | 6.970 |
| C_3 | 6.267 | 6.888 | 6.035 | 6.280 | 6.893 |
| F_9 | 6.203 | 5.687 | 7.148 | 6.546 | 7.141 |
| P_2 | 6.376 | 6.487 | 6.687 | 6.681 | 6.808 |
| AF | C | Control | F | CHS | IP | P | |
|---|---|---|---|---|---|---|---|
| AF | 1 | 0.49 | p < 0.001 | 0.34 | p < 0.01 | p < 0.001 | p < 0.05 |
| C | 0.49 | 1 | p < 0.001 | 0.18 | p < 0.01 | p < 0.001 | p < 0.001 |
| Control | p < 0.001 | p < 0.001 | 1 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| F | 0.34 | 0.18 | p < 0.001 | 1 | p < 0.05 | p < 0.001 | p < 0.05 |
| CHS | p < 0.01 | p < 0.01 | p < 0.001 | p < 0.05 | 1 | p < 0.01 | p < 0.001 |
| IP | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.01 | 1 | p < 0.001 |
| P | p < 0.05 | p < 0.001 | p < 0.001 | p < 0.05 | p < 0.001 | p < 0.001 | 1 |
| Hardness | Foreign Taste | Foreign Odor | Salty Taste | Crust Color | Chewability | Crumb Porosity | Moisture | Typical Aroma | |
|---|---|---|---|---|---|---|---|---|---|
| IP | 4.836 | 4.718 | 3.569 | 5.315 | 4.420 | 3.919 | 4.042 | 4.178 | 4.500 |
| CHS | 3.760 | 4.019 | 3.968 | 5.067 | 3.955 | 4.150 | 4.107 | 6.051 | 4.724 |
| C | 3.625 | 4.165 | 3.552 | 4.074 | 4.657 | 3.397 | 4.777 | 5.754 | 5.247 |
| Control | 4.266 | 2.126 | 1.455 | 5.765 | 2.658 | 3.922 | 3.645 | 4.064 | 6.150 |
| F | 3.391 | 3.633 | 3.464 | 4.224 | 3.296 | 4.574 | 4.690 | 6.484 | 5.438 |
| AF | 3.796 | 3.556 | 3.078 | 4.757 | 5.424 | 4.646 | 5.376 | 5.859 | 5.564 |
| P | 2.734 | 3.319 | 2.861 | 5.223 | 4.709 | 3.880 | 4.839 | 6.879 | 5.857 |
| Appearance | Texture | Aroma | Overall Evaluation | Taste | |
|---|---|---|---|---|---|
| IP | 6.161 | 5.736 | 5.509 | 5.553 | 5.200 |
| P | 5.237 | 5.471 | 6.105 | 5.670 | 5.621 |
| CHS | 5.475 | 5.548 | 5.965 | 6.071 | 6.099 |
| AF | 6.533 | 6.019 | 5.915 | 5.856 | 5.824 |
| Control | 5.654 | 5.743 | 6.436 | 6.258 | 6.210 |
| F | 5.632 | 6.226 | 6.257 | 5.999 | 6.266 |
| C | 6.658 | 5.796 | 5.969 | 6.753 | 6.269 |
| Sample | Firmness (g) |
|---|---|
| F_9 | 3.680 ± 0.328 |
| F_13 | 3.458 ± 0.503 |
| F_17 | 3.803 ± 0.476 |
| F_23 | 4.177 ± 0.680 |
| P_2 | 3.765 ± 0.390 |
| P_5 | 3.547 ± 0.404 |
| P_7 | 3.700 ± 0.315 |
| P_9 | 3.637 ± 0.334 |
| A_2 | 4.435 ± 0.547 |
| A_5 | 4.190 ± 0.415 |
| A_7 | 3.805 ± 0.443 |
| A_9 | 4.163 ± 0.554 |
| CH_2 | 3.778 ± 0.225 |
| CH_5 | 4.280 ± 0.400 |
| CH_9 | 3.882 ± 0.625 |
| CH_13 | 3.970 ± 0.583 |
| I_2 | 3.893 ± 0.338 |
| I_5 | 3.832 ± 0.353 |
| I_9 | 3.792 ± 0.362 |
| I_13 | 4.228 ± 0.657 |
| C_1 | 3.918 ± 0.589 |
| C_2 | 4.035 ± 0.380 |
| C_3 | 3.935 ± 0.360 |
| C_4 | 4.035 ± 0.215 |
| Ctrl | 4.048 ± 0.462 |
| Sample | TPC (mg GAE/100 g) |
|---|---|
| I_9 | 22.29 ± 2.36 a |
| I_13 | 21.11 ± 0.31 ab |
| I_5 | 21.06 ± 0.06 ab |
| I_2 | 20.64 ± 0.16 abc |
| C_4 | 20.53 ± 0.09 abcd |
| C_1 | 20.52 ± 0.01 abcd |
| A_2 | 20.74 ± 0.74 abcd |
| A_9 | 20.45 ± 0.10 abcde |
| C_2 | 20.46 ± 0.20 abcdef |
| F_23 | 20.33 ± 0.06 abcdefg |
| CH_13 | 20.31 ± 0.19 abcdefgh |
| C_3 | 20.31 ± 0.16 abcdefgh |
| CH_2 | 20.48 ± 0.69 abcdefghi |
| F_9 | 20.21 ± 0.09 abcdefghi |
| A_7 | 20.20 ± 0.05 bcdefghi |
| P_7 | 21.08 ± 2.35 cdefghi |
| A_5 | 20.09 ± 0.11 cdefghi |
| F_17 | 20.03 ± 0.14 defghi |
| Ctrl | 20.06 ± 0.06 efghi |
| F_13 | 20.01 ± 0.17 efghi |
| CH_5 | 19.97 ± 0.06 efghi |
| P_9 | 19.69 ± 0.62 fghi |
| P_2 | 19.94 ± 0.08 ghi |
| P_5 | 19.81 ± 0.09 hi |
| CH_9 | 19.72 ± 0.02 i |
| Sample | ABTS (%) | Sample | DPPH (%) |
|---|---|---|---|
| A_9 | 20.39 ± 1.63 | I_2 | 20.92 ± 0.51 |
| A_2 | 19.32 ± 1.57 | I_5 | 20.37 ± 2.33 |
| A_5 | 18.20 ± 3.40 | I_13 | 19.50 ± 1.90 |
| I_13 | 18.10 ± 3.63 | I_9 | 17.39 ± 2.17 |
| I_9 | 17.57 ± 2.68 | C_4 | 15.80 ± 0.95 |
| C_2 | 17.50 ± 3.97 | C_1 | 15.69 ± 0.72 |
| I_2 | 17.44 ± 4.92 | F_9 | 15.57 ± 1.13 |
| I_5 | 17.08 ± 3.81 | A_9 | 15.17 ± 2.39 |
| C_1 | 15.81 ± 1.86 | CH_2 | 14.56 ± 1.37 |
| C_4 | 15.53 ± 2.24 | F_13 | 14.54 ± 2.21 |
| C_3 | 14.47 ± 3.54 | F_23 | 14.51 ± 1.28 |
| F_23 | 14.39 ± 0.97 | C_2 | 14.37 ± 1.27 |
| F_9 | 13.98 ± 0.97 | CH_13 | 14.27 ± 0.87 |
| A_7 | 13.92 ± 1.94 | C_3 | 13.33 ± 1.84 |
| F_13 | 13.81 ± 3.10 | F_17 | 13.30 ± 0.40 |
| F_17 | 12.93 ± 1.36 | Ctrl | 13.29 ± 0.80 |
| CH_2 | 11.01 ± 2.25 | P_2 | 12.80 ± 1.23 |
| CH_13 | 10.55 ± 1.29 | CH_5 | 11.86 ± 2.52 |
| P_2 | 10.29 ± 0.34 | P_5 | 11.16 ± 1.55 |
| Ctrl | 10.10 ± 1.59 | P_7 | 9.32 ± 1.90 |
| P_7 | 10.04 ± 2.54 | A_2 | 9.03 ± 4.06 |
| CH_5 | 9.49 ± 2.45 | CH_9 | 9.01 ± 0.95 |
| P_5 | 9.45 ± 1.59 | P_9 | 8.06 ± 2.37 |
| CH_9 | 8.25 ± 1.65 | A_7 | 6.04 ± 4.19 |
| P_9 | 7.90 ± 2.26 | A_5 | 5.23 ± 4.04 |
| Sample | GI (%) |
|---|---|
| A_5 | 40.61 |
| C_3 | 40.40 |
| C_1 | 40.49 |
| F_9 | 40.72 |
| P_2 | 40.66 |
| Ctrl | 40.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauferová, A.; Pečová, M.; Czerniková, A.; Dordević, D.; Tremlová, B. Effect of Fiber and Insect Powder Addition on Selected Organoleptic and Nutritional Characteristics of Gluten-Free Bread. Processes 2024, 12, 970. https://doi.org/10.3390/pr12050970
Tauferová A, Pečová M, Czerniková A, Dordević D, Tremlová B. Effect of Fiber and Insect Powder Addition on Selected Organoleptic and Nutritional Characteristics of Gluten-Free Bread. Processes. 2024; 12(5):970. https://doi.org/10.3390/pr12050970
Chicago/Turabian StyleTauferová, Alexandra, Martina Pečová, Aneta Czerniková, Dani Dordević, and Bohuslava Tremlová. 2024. "Effect of Fiber and Insect Powder Addition on Selected Organoleptic and Nutritional Characteristics of Gluten-Free Bread" Processes 12, no. 5: 970. https://doi.org/10.3390/pr12050970





