Advanced Antimicrobial and Anti-Infective Strategies to Manage Peri-Implant Infection: A Narrative Review
Abstract
:1. Introduction

2. Methods
2.1. Search Strategy
2.2. Study Selection, Inclusion and Exclusion Criteria
3. Molecular Mechanisms of Implant Colonization by Pathogens
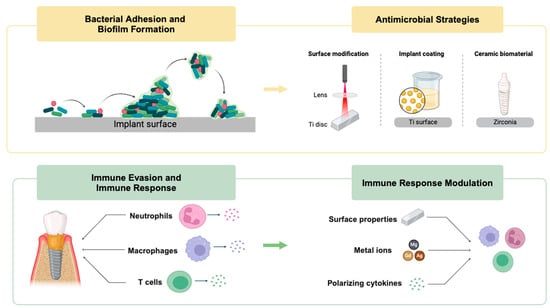
3.1. Bacterial Adhesion and Biofilm Formation
3.2. Immune Evasion
3.3. Modulation of Immune Response

4. Antimicrobial and Anti-Infective Strategies
4.1. Current Materials for Inhibiting Implant Surface Bacterial Adhesion and Biofilm Formation
4.1.1. Physicochemical Surface Modification
4.1.2. Implant Coatings
4.1.3. Ceramic Implant Biomaterials and Other Relevant Therapies
| Category | Material | Mechanism | References |
|---|---|---|---|
| Physicochemical surface modification | UV treatment | Decrease in initial bacterial attachment and subsequent biofilm formation | [64,65,66] |
| Nanotextured surfaces (NTSs) | Bactericidal effect via a combination of strong adhesion and shear force due to topographical changes | [72,73,74] | |
| Implant coatings | Metal nanoparticles | Antimicrobial properties | [87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] |
| Polymer—chitosan | Anti-adhesive and inhibit bacterial attachment | [114] | |
| Ceramic material | Zirconia | Lower surface energy inhibiting bacterial attachment | [131] |
4.2. Current Materials for Regulating the Immune–Inflammatory Response
4.2.1. Modulation of Neutrophils
4.2.2. Modulation of Macrophage Polarization
4.2.3. The Role of T Cells
| Category | Factor | Mechanism | References |
|---|---|---|---|
| Modulation of neutrophils | Surface roughness | Rough surfaces enhance initial neutrophil adherence more efficiently and increase neutrophil death and ROS generation | [142] |
| Surface hydrophilicity | Hydrophilicity significantly reduces the pro-inflammatory activation of leukocytes | [145,146,147] | |
| Surface stiffness | The spread area of neutrophils increases with the rise in matrix stiffness | [149] | |
| Modulation of macrophage | Metal ions (Mg, Gd, Ag) | Promoting the M2 macrophage phenotype | [151,152] |
| polarizing cytokines (IL-4, IL-13, or IL-10) | Activating macrophages into the anti-inflammatory M2 phenotype | [153] | |
| Altering surface properties (nanotubes, AM porous titanium, hydrophilic surfaces) | Inducing pro-regenerative macrophage polarization | [154,155,156,157,158,159] | |
| Modulation of T cells | HAp nanorods | Regulating osteogenesis by modulating T cells and IL-22 during the bone regeneration process | [174] |
5. Conclusions
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis update: Risk indicators, diagnosis, and treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Paauw, D.S. Complications of Antibiotic Therapy. Med. Clin. N. Am. 2013, 97, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.R. Book review: Tackling drug-resistant infections globally. Arch. Pharm. Pract. 2016, 7, 110. [Google Scholar] [CrossRef]
- Belongia, E.A.; Schwartz, B. Strategies for promoting judicious use of antibiotics by doctors and patients. Br. Med. J. 1998, 317, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Palei, A.C.; Spradley, F.T.; Granger, J.P. Role of Nitric Oxide Synthase on Blood Pressure Regulation and Vascular Function in Pregnant Rats on a High-Fat Diet. Am. J. Hypertens. 2017, 30, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T.; Ericsson, I.; Lindhe, J. Spontaneous progression of experimentally induced periimplantitis. J. Clin. Periodontol. 2004, 31, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Abrahamsson, I.; Berglundh, T.; Lindhe, J. Soft tissue reactions to plaque formation at implant abutments with different surface topography: An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 456–461. [Google Scholar] [CrossRef]
- Gualini, F.; Berglundh, T. Immunohistochemical characteristics of inflammatory lesions at implants. J. Clin. Periodontol. 2003, 30, 14–18. [Google Scholar] [CrossRef]
- Lindhe, J.; Berglundh, T.; Ericsson, I.; Liljenberg, B.; Marinello, C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin. Oral Implant. Res. 1992, 3, 9–16. [Google Scholar] [CrossRef]
- Carcuac, O.; Abrahamsson, I.; Albouy, J.P.; Linder, E.; Larsson, L.; Berglundh, T. Experimental periodontitis and peri-implantitis in dogs. Clin. Oral Implant. Res. 2013, 24, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for the treatment of complex bone diseases: Bone cancer, bone infection and osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Bos, R.; Van Der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions—Its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Horswill, A.R. agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Naylor, P.T.; Myrvik, Q.N. Biomaterial-Centered Infections: Microbial Adhesion versus Tissue Integration. Pathog. Wound Biomater. Infect. 1990, 7, 193–216. [Google Scholar] [CrossRef]
- Fowler, T.; Wann, E.R.; Joh, D.; Johansson, S.; Foster, T.J.; Höök, M. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectic-binding MSCRAMMs and host cell β1 integrins. Eur. J. Cell Biol. 2000, 79, 672–679. [Google Scholar] [CrossRef]
- Alexander, E.H.; Rivera, F.A.; Marriott, I.; Anguita, J.; Bost, K.L.; Hudson, M.C. Staphylococcus aureus—Induced tumor necrosis factor—Related apoptosis—Inducing ligand expression mediates apoptosis and caspase-8 activation in infected osteoblasts. BMC Microbiol. 2003, 3, 5. [Google Scholar] [CrossRef]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and Systemic Effects of Porphyromonas gingivalis Infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef]
- Crémet, L.; Broquet, A.; Jacqueline, C.; Chaillou, C.; Asehnoune, K.; Corvec, S.; Caroff, N. Innate immune evasion of Escherichia coli clinical strains from orthopedic implant infections. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Efron, N.; Efron, S.E. Therapeutic Applications. In Contact Lens Practice E-Book; Elsevier: Amsterdam, The Netherlands, 2018; pp. 275–281.e1. [Google Scholar] [CrossRef]
- Tang, L.; Eaton, J.W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 1993, 178, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lucas, A.H.; Eaton, J.W. Inflammatory responses to implanted polymeric biomaterials: Role of surface-adsorbed immunoglobulin G. J. Lab. Clin. Med. 1993, 122, 292–300. [Google Scholar] [PubMed]
- Eriksson, C.; Lausmaa, J.; Nygren, H. Interactions between human whole blood and modified TiO2-surfaces: Influence of surface topography and oxide thickness on leukocyte adhesion and activation. Biomaterials 2001, 22, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Palmer, L.J.; Chapple, I.L.C. Neutrophil extracellular traps as a new paradigm in innate immunity: Friend or foe? Periodontology 2000 2013, 63, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Dadley-Moore, D. SPOT-on malaria target. Nat. Rev. Immunol. 2004, 4, 244. [Google Scholar] [CrossRef]
- GOOD, R.A. Absence of plasma cells from bone marrow and lymph nodes following antigenic stimulation in patients with a gamma globulinemia. Rev. Hematol. 1954, 9, 502–503. [Google Scholar] [PubMed]
- Kovtun, A.; Bergdolt, S.; Wiegner, R.; Radermacher, P.; Huber-Lang, M.; Ignatius, A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur. Cells Mater. 2016, 32, 152–162. [Google Scholar] [CrossRef]
- Berbari, E.F.; Osmon, D.R.; Carr, A.; Hanssen, A.D.; Baddour, L.M.; Greene, D.; Kupp, L.I.; Baughan, L.W.; Scott Harmsen, W.; Mandrekar, J.N.; et al. Dental procedures as risk factors for prosthetic hip or knee infection: A hospital-based prospective case-control study. Clin. Infect. Dis. 2010, 50, 8–16. [Google Scholar] [CrossRef]
- Rigby, K.M.; DeLeo, F.R. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 2012, 34, 237–259. [Google Scholar] [CrossRef]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Bernard, L.; Vaudaux, P.; Huggler, E.; Stern, R.; Fréhel, C.; Francois, P.; Lew, D.; Hoffmeyer, P. Inactivation of a subpopulation of human neutrophils by exposure to ultrahigh-molecular-weight polyethylene wear debris. FEMS Immunol. Med. Microbiol. 2007, 49, 425–432. [Google Scholar] [CrossRef]
- Neth, O.W.; Bajaj-Elliott, M.; Turner, M.W.; Klein, N.J. Susceptibility to infection in patients with neutropenia: The role of the innate immune system. Br. J. Haematol. 2005, 129, 713–722. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Simkin, J.; Dawson, L.A.; Simkin, M.; Muneoka, K. Healing power: The mammalian macrophage in skeletal regeneration, scar formation, and regenerative medicine. J. Immunol. Regen. Med. 2020, 7, 100026. [Google Scholar] [CrossRef]
- Wu, C.L.; Harasymowicz, N.S.; Klimak, M.A.; Collins, K.H.; Guilak, F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr. Cartil. 2020, 28, 544–554. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gibon, E.; Pajarinen, J.; Lin, T.H.; Keeney, M.; Ren, P.G.; Nich, C.; Yao, Z.; Egashira, K.; Yang, F.; et al. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J. R. Soc. Interface 2014, 11, 20130962. [Google Scholar] [CrossRef] [PubMed]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. Npj Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Bone immune response to materials, part I: Titanium, peek and copper in comparison to sham at 10 days in rabbit tibia. J. Clin. Med. 2018, 7, 526. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Bone immune response to materials, Part II: Copper and polyetheretherketone (PEEK) compared to titanium at 10 and 28 days in rabbit tibia. J. Clin. Med. 2019, 8, 814. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, Y.; Zhang, Y.; Ruan, Z. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann. Clin. Lab. Sci. 2016, 46, 65–71. [Google Scholar] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, Y.; Wei, S. M2 macrophages are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury: A retrospective cohort study. J. Orthop. Surg. Res. 2018, 13, 213. [Google Scholar] [CrossRef]
- Cosin-Roger, J.; Ortiz-Masià, M.D.; Barrachina, M.D. Macrophages as an emerging source of wnt ligands: Relevance in mucosal integrity. Front. Immunol. 2019, 10, 2297. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Origins and Hallmarks of Macrophages: Development, Homeostasis, and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, X.; Huang, L.; Mouraret, S.; Brunski, J.B.; Cordova, L.; Salmon, B.; Helms, J.A. Relationships among Bone Quality, Implant Osseointegration, and Wnt Signaling. J. Dent. Res. 2017, 96, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2019, 6, 170. [Google Scholar] [CrossRef]
- Stoick-Cooper, C.L.; Weidinger, G.; Riehle, K.J.; Hubbert, C.; Major, M.B.; Fausto, N.; Moon, R.T. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 2007, 134, 479–489. [Google Scholar] [CrossRef]
- Alexander, K.A.; Raggatt, L.J.; Millard, S.; Batoon, L.; Chiu-Ku Wu, A.; Chang, M.K.; Hume, D.A.; Pettit, A.R. Resting and injury-induced inflamed periosteum contain multiple macrophage subsets that are located at sites of bone growth and regeneration. Immunol. Cell Biol. 2017, 95, 7–16. [Google Scholar] [CrossRef]
- Yang, D.H.; Yang, M.Y. The role of macrophage in the pathogenesis of osteoporosis. Int. J. Mol. Sci. 2019, 20, 2093. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng.-Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines 2018, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Mescher, A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration 2017, 4, 39–53. [Google Scholar] [CrossRef]
- Wendler, S.; Schlundt, C.; Bucher, C.H.; Birkigt, J.; Schipp, C.J.; Volk, H.D.; Duda, G.N.; Schmidt-Bleek, K. Immune modulation to enhance bone healing-a new concept to induce bone using prostacyclin to locally modulate immunity. Front. Immunol. 2019, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef] [PubMed]
- Limmer, A.; Wirtz, D.C. Osteoimmunology: Influence of the Immune System on Bone Regeneration and Consumption. Z. Orthop. Unfallchirurgie 2017, 155, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Seebach, E.; Kubatzky, K.F. Chronic Implant-Related Bone Infections-Can Immune Modulation be a Therapeutic Strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of dental prosthesis and restorative materials interface on oral biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef]
- Hahnel, S.; Wieser, A.; Lang, R.; Rosentritt, M. Biofilm formation on the surface of modern implant abutment materials. Clin. Oral Implant. Res. 2015, 26, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.d.; Pita, M.S.; Fernandes, F.H.N.C.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin. Oral Implant. Res. 2014, 25, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Funato, A.; Yamada, M.; Ogawa, T. Success rate, healing time, and implant stability of photofunctionalized dental implants. Int. J. Oral Maxillofac. Implants 2013, 28, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Funato, A.; Ogawa, T. Photofunctionalized dental implants: A case series in compromised bone. Int. J. Oral Maxillofac. Implants 2013, 28, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Tsukimura, N.; Yamada, M.; Iwasa, F.; Minamikawa, H.; Att, W.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials 2011, 32, 4358–4368. [Google Scholar] [CrossRef]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J. Biomed. Mater. Res. A 2014, 102, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Yamada, M.; Yamamoto, A.; Iwasa, F.; Suzawa, T.; Kamijo, R.; Baba, K.; Ogawa, T. The enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfaces. Biomaterials 2010, 31, 3827–3839. [Google Scholar] [CrossRef]
- Pyo, S.W.; Park, Y.B.; Moon, H.S.; Lee, J.H.; Ogawa, T. Photofunctionalization enhances bone-implant contact, dynamics of interfacial osteogenesis, marginal bone seal, and removal torque value of implants: A dog jawbone study. Implant Dent. 2013, 22, 666–675. [Google Scholar] [CrossRef]
- Sugita, Y.; Honda, Y.; Kato, I.; Kubo, K.; Maeda, H.; Ogawa, T. Role of photofunctionalization in mitigating impaired osseointegration associated with type 2 diabetes in rats. Int. J. Oral Maxillofac. Implants 2014, 29, 1293–1300. [Google Scholar] [CrossRef]
- Pesce, P.; Menini, M.; Santori, G.; Giovanni, E.; Bagnasco, F.; Canullo, L. Photo and Plasma Activation of Dental Implant Titanium Surfaces. A Systematic Review with Meta-Analysis of Pre-Clinical Studies. J. Clin. Med. 2020, 9, 2817. [Google Scholar] [CrossRef]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; Yee, A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, C.M.; Khanh Truong, V.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bhadra, C.M.; Yen Dang, T.H.; Buividas, R.; Wang, J.; Crawford, R.J.; Ivanova, E.P.; Juodkazis, S. A bactericidal microfluidic device constructed using nano-textured black silicon. RSC Adv. 2016, 6, 26300–26306. [Google Scholar] [CrossRef]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, Å.; Dahlén, G. Effect of titanium on selected oral bacterial species in vitro. Eur. J. Oral Sci. 1995, 103, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K.; Hirayama, A. Influence of surface modifications to titanium on oral bacterial adhesion in vitro. J. Biomed. Mater. Res. 2000, 52, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Qiao, Y.; Liu, X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Junkar, I.; Kulkarni, M.; Benčina, M.; Kovač, J.; Mrak-Poljšak, K.; Lakota, K.; Sodin-Šemrl, S.; Mozetič, M.; Iglič, A. Titanium Dioxide Nanotube Arrays for Cardiovascular Stent Applications. ACS Omega 2020, 5, 7280–7289. [Google Scholar] [CrossRef]
- Wang, C.; Huang, X.; Deng, W.; Chang, C.; Hang, R.; Tang, B. A nano-silver composite based on the ion-exchange response for the intelligent antibacterial applications. Mater. Sci. Eng. C 2014, 41, 134–141. [Google Scholar] [CrossRef]
- Jin, X.; Gao, L.; Liu, E.; Yu, F.; Shu, X.; Wang, H. Microstructure, corrosion and tribological and antibacterial properties of Ti-Cu coated stainless steel. J. Mech. Behav. Biomed. Mater. 2015, 50, 23–32. [Google Scholar] [CrossRef]
- Balestriere, M.A.; Schuhladen, K.; Herrera Seitz, K.; Boccaccini, A.R.; Cere, S.M.; Ballarre, J. Sol-gel coatings incorporating borosilicate bioactive glass enhance anti corrosive and surface performance of stainless steel implants. J. Electroanal. Chem. 2020, 876, 114735. [Google Scholar] [CrossRef]
- Hui, R.; Wang, Z.; Kesler, O.; Rose, L.; Jankovic, J.; Yick, S.; Maric, R.; Ghosh, D. Thermal plasma spraying for SOFCs: Applications, potential advantages, and challenges. J. Power Sources 2007, 170, 308–323. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, A.; Vardelle, M. Modelling of plasma spraying of ceramic coatings at atmospheric pressure. Ceram. Int. 1991, 17, 367–379. [Google Scholar] [CrossRef]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J. Mater. Chem. B 2015, 3, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Cao, H.; Zhao, Y.; Jin, G.; Cheng, M.; Wang, J.; Jiang, Y.; An, Z.; Zhang, X.; Liu, X. Antimicrobial and Osteogenic Properties of Silver-Ion-Implanted Stainless Steel. ACS Appl. Mater. Interfaces 2015, 7, 10785–10794. [Google Scholar] [CrossRef]
- Bai, L.; Hang, R.; Gao, A.; Zhang, X.; Huang, X.; Wang, Y.; Tang, B.; Zhao, L.; Chu, P.K. Nanostructured titanium-silver coatings with good antibacterial activity and cytocompatibility fabricated by one-step magnetron sputtering. Appl. Surf. Sci. 2015, 355, 32–44. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, H.; Qiao, S.; Wang, M.; Gu, Y.; Luo, H.; Meng, F.; Liu, X.; Lai, H. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659–6674. [Google Scholar] [CrossRef]
- Qiao, S.; Cao, H.; Zhao, X.; Lo, H.; Zhuang, L.; Gu, Y.; Shi, J.; Liu, X.; Lai, H. Ag-plasma modification enhances bone apposition around titanium dental implants: An animal study in labrador dogs. Int. J. Nanomed. 2015, 10, 653–664. [Google Scholar] [CrossRef]
- Sarraf, M.; Dabbagh, A.; Abdul Razak, B.; Mahmoodian, R.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Abu Kasim, N.H.; Abdullah, H.; Sukiman, N.L. Highly-ordered TiO2 nanotubes decorated with Ag2O nanoparticles for improved biofunctionality of Ti6Al4V. Surf. Coat. Technol. 2018, 349, 1008–1017. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Huo, K.; Gao, B.; Xiong, W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J. Biomed. Mater. Res.-Part A 2014, 102, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, A.; Bai, L.; Wang, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B.; Chu, P.K. Antibacterial, osteogenic, and angiogenic activities of SrTiO3 nanotubes embedded with Ag2O nanoparticles. Mater. Sci. Eng. C 2017, 75, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Sarraf, M.; Dabbagh, A.; Abdul Razak, B.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Abu Kasim, N.H.; Yean, L.K.; Sukiman, N.L. Silver oxide nanoparticles-decorated tantala nanotubes for enhanced antibacterial activity and osseointegration of Ti6Al4V. Mater. Des. 2018, 154, 28–40. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Ge, S.; Chen, J.; Ji, P. The relationship between substrate morphology and biological performances of nano-silver-loaded dopamine coatings on titanium surfaces. R Soc. Open Sci. 2018, 5, 172310. [Google Scholar] [CrossRef]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res.-Part A 2017, 105, 2218–2227. [Google Scholar] [CrossRef]
- Fernández-Villa, D.; Gómez-Lavín, M.J.; Abradelo, C.; Román, J.S.; Rojo, L. Tissue engineering therapies based on folic acid and other vitamin B derivatives. Functional mechanisms and current applications in regenerative medicine. Int. J. Mol. Sci. 2018, 19, 4068. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Balancing Bacteria-Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine Nanorods. ACS Nano 2017, 11, 11250–11263. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Li, S.; Guo, S.; He, M.; Luo, K.; Lin, J. Copper-modified Ti6Al4V alloy fabricated by selective laser melting with pro-angiogenic and anti-inflammatory properties for potential guided bone regeneration applications. Mater. Sci. Eng. C 2018, 90, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Moreira, H.; Costa-Barbosa, A.; Marques, S.M.; Sampaio, P.; Carvalho, S. Evaluation of cell activation promoted by tantalum and tantalum oxide coatings deposited by reactive DC magnetron sputtering. Surf. Coat. Technol. 2017, 330, 260–269. [Google Scholar] [CrossRef]
- Veerachamy, S.; Hameed, P.; Sen, D.; Dash, S.; Manivasagam, G. Studies on Mechanical, Biocompatibility and Antibacterial Activity of Plasma Sprayed Nano/Micron Ceramic Bilayered Coatings on Ti–6Al–4V Alloy for Biomedical Application. J. Nanosci. Nanotechnol. 2017, 18, 4515–4523. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, G.; Liu, X.; Sun, G.; Li, D.; Wei, H. A decomposable silica-based antibacterial coating for percutaneous titanium implant. Int. J. Nanomed. 2017, 12, 371–379. [Google Scholar] [CrossRef]
- Costa, B.C.; Rodrigues, E.A.; Tokuhara, C.K.; Oliveira, R.C.; Lisboa-Filho, P.N.; Rocha, L.A. ZnO Nanoparticles with Different Sizes and Morphologies for Medical Implant Coatings: Synthesis and Cytotoxicity. Bionanoscience 2018, 8, 587–595. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Koegel, S.; Waltimo, T.; Zimmermann, A.; Walker, C.; Hauser-Gerspach, I.; Jung, C. Antimicrobial efficacy of copper-doped titanium surfaces for dental implants. J. Mater. Sci. Mater. Med. 2019, 30, 84. [Google Scholar] [CrossRef]
- Di, H.; Qiaoxia, L.; Yujie, Z.; Jingxuan, L.; Yan, W.; Yinchun, H.; Xiaojie, L.; Song, C.; Weiyi, C. Ag nanoparticles incorporated tannic acid/nanoapatite composite coating on Ti implant surfaces for enhancement of antibacterial and antioxidant properties. Surf. Coat. Technol. 2020, 399, 126169. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.; Roh, J.; Kim, S.; Choi, K.; Yi, J.; Kim, Y.; Yoon, J. Biofilm-inactivating activity of silver nanoparticles: A comparison with silver ions. J. Ind. Eng. Chem. 2013, 19, 614–619. [Google Scholar] [CrossRef]
- Durner, J.; Stojanovic, M.; Urcan, E.; Hickel, R.; Reichl, F.X. Influence of silver nano-particles on monomer elution from light-cured composites. Dent. Mater. 2011, 27, 631–636. [Google Scholar] [CrossRef]
- Rojo, L.; Deb, S. Polymer Therapeutics in Relation to Dentistry. Front. Oral Biol. 2015, 17, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef]
- Sollazzo, V.; Pezzetti, F.; Scarano, A.; Piattelli, A.; Bignozzi, C.A.; Massari, L.; Brunelli, G.; Carinci, F. Zirconium oxide coating improves implant osseointegration in vivo. Dent. Mater. 2008, 24, 357–361. [Google Scholar] [CrossRef]
- Knabe, C.; Klar, F.; Fitzner, R.; Radlanski, R.J.; Gross, U. In vitro investigation of titanium and hydroxyapatite dental implant surfaces using a rat bone marrow stromal cell culture system. Biomaterials 2002, 23, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Darimont, G.L.; Cloots, R.; Heinen, E.; Seidel, L.; Legrand, R. In vivo behaviour of hydroxyapatite coatings on titanium implants: A quantitative study in the rabbit. Biomaterials 2002, 23, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Ochsenbein, A.; Chai, F.; Winter, S.; Traisnel, M.; Breme, J.; Hildebrand, H.F. Osteoblast responses to different oxide coatings produced by the sol-gel process on titanium substrates. Acta Biomater. 2008, 4, 1506–1517. [Google Scholar] [CrossRef]
- Simon, M.; Lagneau, C.; Moreno, J.; Lissac, M.; Dalard, F.; Corrosion, G.B.; Simon, M.; Moreno, J. Corrosion resistance and biocompatibility of a new porous surface for titanium implants. Eur. J. Oral Sci. 2005, 113, 537–545. [Google Scholar] [CrossRef]
- Rong, M.; Zhou, L.; Gou, Z.; Zhu, A.; Zhou, D. The early osseointegration of the laser-treated and acid-etched dental implants surface: An experimental study in rabbits. J. Mater. Sci. Mater. Med. 2009, 20, 1721–1728. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, Z.; Dai, Q.X.; Lin, D.Y.; Li, S.M. Microstructure and bond strength of HA(+ZrO2 + Y2O3)/Ti6Al4V composite coatings fabricated by RF magnetron sputtering. Surf. Coat. Technol. 2006, 200, 5354–5363. [Google Scholar] [CrossRef]
- Aparicio, C.; Rodriguez, D.; Gil, F.J. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 2011, 31, 320–324. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Barber, Z.H.; Best, S.M.; Bonfield, W. Surface modification of magnetron-sputtered hydroxyapatite thin films via silicon substitution for orthopaedic and dental applications. Surf. Coat. Technol. 2011, 205, 3472–3477. [Google Scholar] [CrossRef]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Hatton, P.V.; Le Maitre, C.; Smith, T.J.; Akid, R. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated titanium. Bone Jt. J. 2021, 103 B, 522–529. [Google Scholar] [CrossRef]
- He, F.M.; Yang, G.L.; Li, Y.N.; Wang, X.X.; Zhao, S.F. Early bone response to sandblasted, dual acid-etched and H2O2/HCl treated titanium implants: An experimental study in the rabbit. Int. J. Oral Maxillofac. Surg. 2009, 38, 677–681. [Google Scholar] [CrossRef]
- Dion, I.; Bordenave, L.; Lefebvre, F.; Bareille, R.; Baquey, C.; Monties, J.R.; Havlik, P. Physico-chemistry and cytotoxicity of ceramics—Part II Cytotoxicity of ceramics. J. Mater. Sci. Mater. Med. 1994, 5, 18–24. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lee, T.M.; Lu, Y.Z.; Yang, C.W.; Lui, T.S.; Kuo, A.; Huang, B.W. The influence of plasma-spraying parameters on the characteristics of fluorapatite coatings. J. Med. Biol. Eng. 2010, 30, 91–98. [Google Scholar]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial Adhesion on Commercially Pure Titanium and Zirconium Oxide Disks: An In Vivo Human Study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef]
- Nakamura, K.; Kanno, T.; Milleding, P.; Ortengren, U. Zirconia as a dental implant abutment material: A systematic review. Int. J. Prosthodont. 2010, 23, 299–309. [Google Scholar]
- Do Nascimento, C.; Pita, M.S.; Santos, E.D.S.; Monesi, N.; Pedrazzi, V.; De Albuquerque Junior, R.F.; Ribeiro, R.F. Microbiome of titanium and zirconia dental implants abutments. Dent. Mater. 2016, 32, 93–101. [Google Scholar] [CrossRef]
- Zhao, B.; Van Der Mei, H.C.; Subbiahdoss, G.; De Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef]
- Apratim, A.; Eachempati, P.; Krishnappa Salian, K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 147. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Fletcher, P.; Deluiz, D.; Tinoco, E.M.; Ricci, J.L.; Tarnow, D.P.; Tinoco, J.M. Human Histologic Evidence of Reosseointegration Around an Implant Affected with Peri-implantitis Following Decontamination with Sterile Saline and Antiseptics: A Case History Report. Int. J. Periodontics Restorative Dent. 2017, 37, 499–508. [Google Scholar] [CrossRef]
- Candotto, V.; Gabrione, F.; Oberti, L.; Lento, D.; Severino, M. The role of implant-abutment connection in preventing bacterial leakage: A review. J. Biol. Regul. Homeost. Agents 2019, 33, 129–134. [Google Scholar]
- Schmitt, C.M.; Nogueira-Filho, G.; Tenenbaum, H.C.; Lai, J.Y.; Brito, C.; Döring, H.; Nonhoff, J. Performance of conical abutment (Morse Taper) connection implants: A systematic review. J. Biomed. Mater. Res. A 2014, 102, 552–574. [Google Scholar] [CrossRef]
- Rodrigues, V.V.M.; Faé, D.S.; Rosa, C.D.D.R.D.; Bento, V.A.A.; Lacerda, M.F.L.S.; Pellizzer, E.P.; Lemos, C.A.A. Is the clinical performance of internal conical connection better than internal non-conical connection for implant-supported restorations? A systematic review with meta-analysis of randomized controlled trials. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2023, 32, 382–391. [Google Scholar] [CrossRef]
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater. Sci. Eng. C 2020, 108, 110411. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness☆. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef]
- Campos, V.; Melo, R.C.N.; Silva, L.P.; Aquino, E.N.; Castro, M.S.; Fontes, W. Characterization of neutrophil adhesion to different titanium surfaces. Bull. Mater. Sci. 2014, 37, 157–166. [Google Scholar] [CrossRef]
- Ley, K. M1 Means Kill; M2 Means Heal. J. Immunol. 2017, 199, 2191–2193. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Musselman, R.M.; Olivares-Navarrete, R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 2020, 8, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Clark, N.M.; Olivares-Navarrete, R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 2018, 182, 202–215. [Google Scholar] [CrossRef]
- Dai, X.; Wei, Y.; Zhang, X.; Meng, S.; Mo, X.; Liu, X.; Deng, X.; Zhang, L.; Deng, X. Attenuating Immune Response of Macrophage by Enhancing Hydrophilicity of Ti Surface. J. Nanomater. 2015, 2015, 712810. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Olivares-Navarrete, R. Substrate stiffness induces neutrophil extracellular trap (NET) formation through focal adhesion kinase activation. Biomaterials 2021, 271, 120715. [Google Scholar] [CrossRef]
- Oakes, P.W.; Patel, D.C.; Morin, N.A.; Zitterbart, D.P.; Fabry, B.; Reichner, J.S.; Tang, J.X. Neutrophil morphology and migration are affected by substrate elasticity. Blood 2009, 114, 1387–1395. [Google Scholar] [CrossRef]
- Hachim, D.; Lopresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef]
- dos Santos Corpas, L.; Lambrichts, I.; Quirynen, M.; Collaert, B.; Politis, C.; Vrielinck, L.; Martens, W.; Struys, T.; Jacobs, R. Peri-implant bone innervation: Histological findings in humans. Eur. J. Oral Implantol. 2014, 7, 283–292. [Google Scholar]
- Costantino, M.D.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J.C. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Zhang, R.; Pajarinen, J.; Lin, T.H.; Goodman, S.B.; Yao, Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 2016, 7, 15. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Song, W.; Jin, J.; Ma, Q.; Fei, D.; Fang, L.; Chen, L.; Wang, Q.; Zhang, Y. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int. J. Nanomed. 2018, 13, 4029–4043. [Google Scholar] [CrossRef]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef]
- Gao, S.; Lu, R.; Wang, X.; Chou, J.; Wang, N.; Huai, X.; Wang, C.; Zhao, Y.; Chen, S. Immune response of macrophages on super-hydrophilic TiO2 nanotube arrays. J. Biomater. Appl. 2020, 34, 1239–1253. [Google Scholar] [CrossRef]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100B, 9–16. [Google Scholar] [CrossRef]
- Razzi, F.; Fratila-Apachitei, L.E.; Fahy, N.; Bastiaansen-Jenniskens, Y.M.; Apachitei, I.; Farrell, E.; Zadpoor, A.A. Immunomodulation of surface biofunctionalized 3D printed porous titanium implants. Biomed. Mater. 2020, 15, 035017. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Ayad, N.B.; Hyzy, S.L.; Boyan, B.D.; Olivares-Navarrete, R. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin. Oral Implant. Res. 2017, 28, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Mardegan, G.P.; Shibli, J.A.; Roth, L.A.; Faveri, M.; Giro, G.; Bastos, M.F. Transforming growth factor-β, interleukin-17, and IL-23 gene expression profiles associated with human peri-implantitis. Clin. Oral Implant. Res. 2017, 28, e10–e15. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. (1986) 2005, 175, 5–14. [Google Scholar] [CrossRef]
- Glimcher, L.H.; Murphy, K.M. Lineage commitment in the immune system: The T helper lymphocyte grows up. Genes Dev. 2000, 14, 1693–1711. [Google Scholar] [CrossRef]
- Dong, C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008, 8, 337–348. [Google Scholar] [CrossRef]
- Martinez, G.J.; Nurieva, R.I.; Yang, X.O.; Dong, C. Regulation and function of proinflammatory TH17 cells. Ann. N. Y. Acad. Sci. 2008, 1143, 188–211. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Hoffman, R.M. Application of GFP imaging in cancer. Lab. Investig. 2015, 95, 432–452. [Google Scholar] [CrossRef]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K.; Lindén, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Wang, J.Y.; Tsukayama, D.T.; Wicklund, B.H.; Gustilo, R.B. Inhibition of T and B cell proliferation by titanium, cobalt, and chromium: Role of IL-2 and IL-6. J. Biomed. Mater. Res. 1996, 32, 655–661. [Google Scholar] [CrossRef]
- Heyman, O.; Koren, N.; Mizraji, G.; Capucha, T.; Wald, S.; Nassar, M.; Tabib, Y.; Shapira, L.; Hovav, A.H.; Wilensky, A. Impaired differentiation of Langerhans cells in the murine oral epithelium adjacent to titanium dental implants. Front. Immunol. 2018, 9, 1712. [Google Scholar] [CrossRef]
- Chen, Z.; Yuen, J.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate. Biomaterials 2015, 61, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lian, R.; Liu, L.; Liu, T.; Bi, C.; Hong, K.; Zhang, S.; Ren, J.; Wang, H.; Ouyang, N.; et al. Biomimetic Hydroxyapatite Nanorods Promote Bone Regeneration via Accelerating Osteogenesis of BMSCs through T Cell-Derived IL-22. ACS Nano 2022, 16, 755–770. [Google Scholar] [CrossRef] [PubMed]
| Database | PubMed, Google Scholar, Embase, Cochrane Library |
|---|---|
| Publication date | Until December 2023 |
| Keywords | “bacterial adhesion”, “biofilm formation”, “antimicrobial”, “neutrophils”, “macrophages”, “T cells”, “immune evasion”, “immune modulation”, and “peri-implant infection” |
| Language | English |
| Type of paper | In vitro studies, in vivo studies, clinical studies, reviews, systematic reviews |
| Inclusion criteria | Articles relating to main focuses with similar materials and methods |
| Exclusion criteria | (1) Non-English-language articles, books, other types of articles; (2) Studies not specifically addressing the characteristics of dental implant surfaces. |
| Journal category | All |
| Method | Species | Mechanism | Reference |
|---|---|---|---|
| Invading host cells | S. aureus | Fibronectin | [18] |
| Combating host immune defences | P. gingivalis | Gingipain R (Rgp), immune response disruption | [20] |
| E. coli | Increased resistance to complement | [21] | |
| A. actinomycetemcomitans | Leukotoxin (LTX), immune cell damage | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Stewart, C.A.; Finer, Y. Advanced Antimicrobial and Anti-Infective Strategies to Manage Peri-Implant Infection: A Narrative Review. Dent. J. 2024, 12, 125. https://doi.org/10.3390/dj12050125
Li Y, Stewart CA, Finer Y. Advanced Antimicrobial and Anti-Infective Strategies to Manage Peri-Implant Infection: A Narrative Review. Dentistry Journal. 2024; 12(5):125. https://doi.org/10.3390/dj12050125
Chicago/Turabian StyleLi, Yihan, Cameron A. Stewart, and Yoav Finer. 2024. "Advanced Antimicrobial and Anti-Infective Strategies to Manage Peri-Implant Infection: A Narrative Review" Dentistry Journal 12, no. 5: 125. https://doi.org/10.3390/dj12050125