Enhancing Fresh-Cut Apple Preservation: Impact of Slightly Acidic Electrolyzed Water and Chitosan–Apple Essence Microencapsulation Coating on Browning and Flavor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. CH–AEM Coating Solution Preparation
2.3. Treatments and Storing of Fresh-Cut Apple
2.4. Respiration Rate, Weight Loss, Firmness, and Ion Leakage Determination
2.5. Color and Browning Index Determination
2.6. Juice Yield, Total Soluble Solids (TSS), pH, and Titratable Acidity (TA) Determination
2.7. E-Nose Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Fresh-Cut Apple during Storage
3.1.1. Respiratory Rate
3.1.2. Weight Loss
3.1.3. Firmness
3.1.4. Ion Leakage
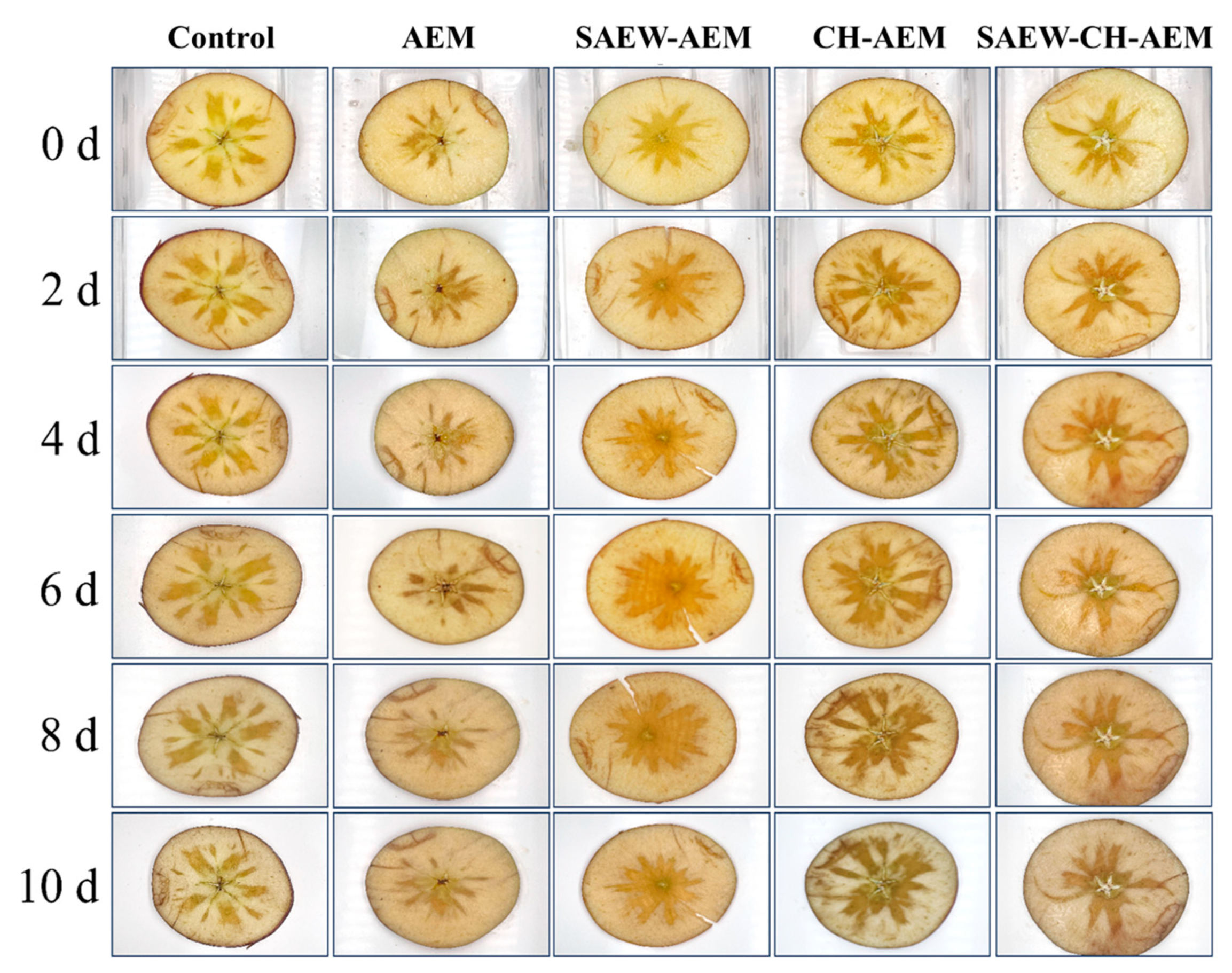
3.2. Color Characteristics of Fresh-Cut Apple during Storage
3.2.1. Sugar Heart
3.2.2. Non-Sugar Heart
3.3. Taste Characteristics of Fresh-Cut Apple during Storage
3.3.1. Juice Yield and TSS
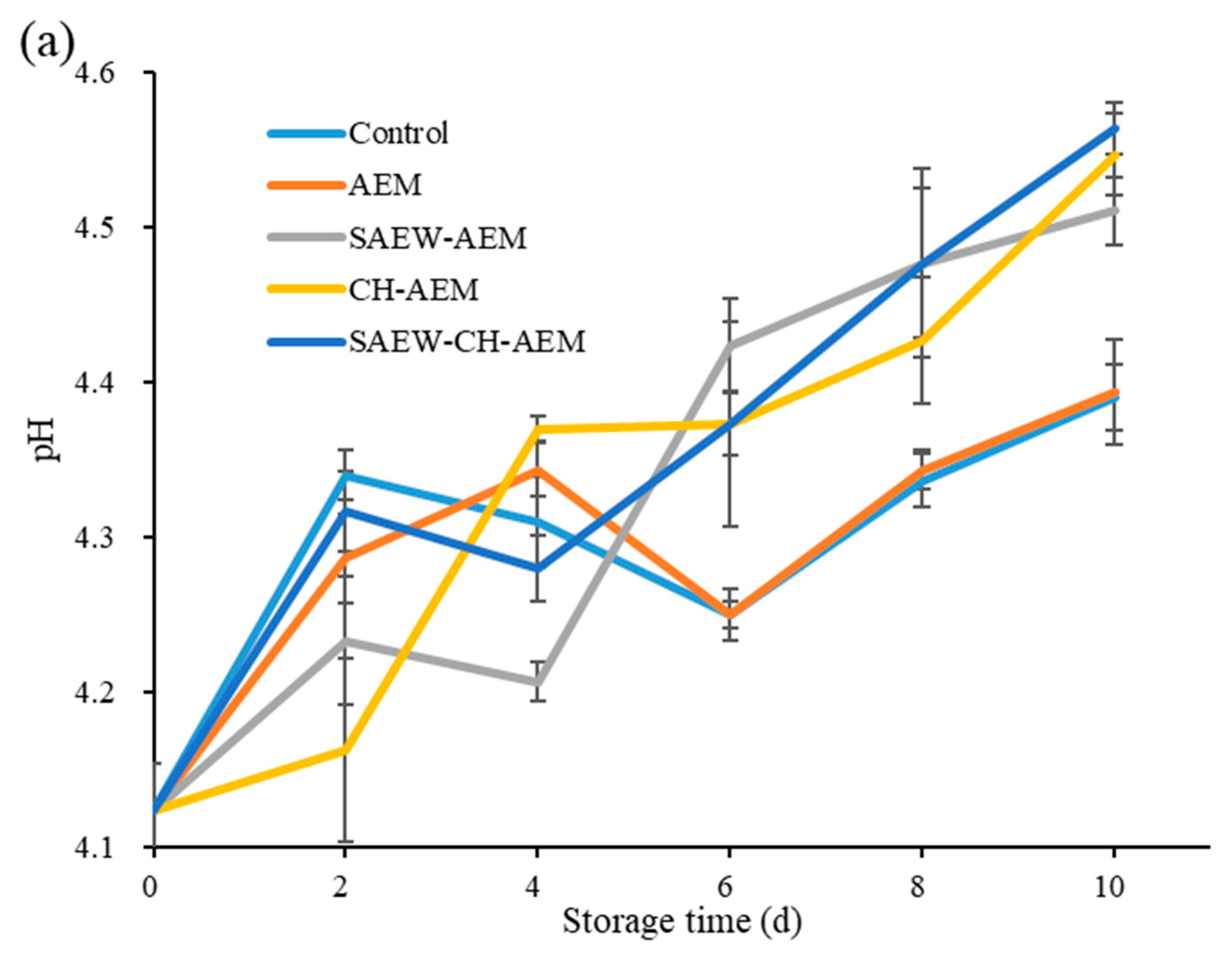
3.3.2. pH and TA
3.4. Aroma Characteristics of Fresh-Cut Apple during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Jiang, A.; Liu, C.; Wagstaffb, C.; Zhao, Q.; Zhang, Y.; Hu, W. Hydrogen sulfide inhibits the browning of fresh-cut apple by regulating the antioxidant, energy and lipid metabolism. Postharvest Biol. Technol. 2021, 175, 111487. [Google Scholar] [CrossRef]
- Salazar-Zúñiga, M.N.; Lugo-Cervantes, E.; Rodríguez-Campos, J.; Sanchez-Vega, R.; Rodríguez-Roque, M.J.; Valdivia-Nájar, C.G. Pulsed Light Processing in the Preservation of Juices and Fresh-Cut Fruits: A Review. Food Bioprocess Technol. 2022, 16, 510–525. [Google Scholar] [CrossRef]
- Mouzahim, M.E.; Eddarai, E.M.; Eladaoui, S.; Guenbour, A.; Bellaouchou, A.; Zarrouk, A.; Boussen, R. Effect of Kaolin clay and Ficus carica mediated silver nanoparticles on chitosan food packaging film for fresh apple slice preservation. Food Chem. 2023, 410, 135470. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Li, X.; Wang, S.; Su, Z.; Sun, H.; Wang, J.; Zhang, W. Phytochemicals-based edible coating for photodynamic preservation of fresh-cut apples. Food Res. Int. 2023, 163, 112293. [Google Scholar] [CrossRef]
- Xin, Y.; Yang, C.; Zhang, J.; Xiong, L. Application of Whey Protein-Based Emulsion Coating Treatment in Fresh-Cut Apple Preservation. Foods 2023, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Wang, D.; Wang, X.; Zhang, F.; Chang, D.; You, C.; Zhang, S.; Wang, X. Effects of cellulose nanofibrils treatment on antioxidant properties and aroma of fresh-cut apples. Food Chem. 2023, 415, 135797. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-L.; Liu, T.-T.; Mao, X.-M.; Quan, X.-Y.; Sui, S.-Y.; Ma, J.-J.; Sun, L.-X.; Li, H.-C.; Shao, Q.-S.; Wang, Y.-N. Insights into the volatile flavor and quality profiles of loquat (Eriobotrya japonica Lindl.) during shelf-life via HS-GC-IMS, E-nose, and E-tongue. Food Chem X 2023, 20, 100886. [Google Scholar] [CrossRef]
- Sun, Z.; Deng, Y.; Zhao, Y. Preparation, Optimization, and Characterization of Natural Apple Essence-Loaded Liposomes. J. Food Sci. 2019, 84, 540–547. [Google Scholar] [CrossRef]
- Demircan, B.; Velioglu, Y.S. Control of Browning, Enzyme Activity, and Quality in Stored Fresh-cut Fruit Salads through Chitosan Coating Enriched with Bergamot Juice Powder. Foods 2024, 13, 147. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Zhang, F.; Tang, Y.; Gong, D.; Oyom, W.; Li, Y.; Prusky, D.; Romanazzi, G.; Bi, Y. Chitosan and chitooligosaccharide regulated reactive oxygen species homeostasis at wounds of pear fruit during healing. Int. J. Biol. Macromol. 2023, 240, 124395. [Google Scholar] [CrossRef]
- Ma, S.; Ma, J.; Wang, Q.; Wang, C.; Shi, H.; Shi, J. NaCl treatment with low density polyethylene packaging bags can enhance the aroma of fresh-cut apple, not only inhibit its browning. Food Packag. Shelf Life 2023, 39, 101148. [Google Scholar] [CrossRef]
- Tappi, S.; Ragni, L.; Tylewicz, U.; Romani, S.; Ramazzina, I.; Rocculi, P. Browning response of fresh-cut apples of different cultivars to cold gas plasma treatment. Innov. Food Sci. Emerg. Technol. 2019, 53, 56–62. [Google Scholar] [CrossRef]
- Fan, X. Chemical inhibition of polyphenol oxidase and cut surface browning of fresh-cut apples. Crit. Rev. Food Sci. Nutr. 2022, 63, 8737–8751. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qiu, W.; Fang, X.; Zhao, X.; Xu, X.; Li, W. Hydrogen-Rich Water Treatment of Fresh-Cut Kiwifruit with Slightly Acidic Electrolytic Water: Influence on Antioxidant Metabolism and Cell Wall Stability. Foods 2023, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Chelliah, R.; Shin, S.; Park, S.; Oh, D.-H.; Wang, M.-H. Slightly acidic electrolyzed water combination with antioxidants and fumaric acid treatment to maintain the quality of fresh-cut bell peppers. LWT 2021, 147, 111565. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Bi, B.; He, J. Effects of Slightly Acidic Electrolyzed Water on the Quality of Fresh-Cut Apple. Foods 2023, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, J.; Jiang, W. Application of electrolyzed water in postharvest fruits and vegetables storage: A review. Trends Food Sci. Technol. 2021, 114, 599–607. [Google Scholar] [CrossRef]
- Li, H.; Lin, L.; Feng, Y.; Zhao, M. Exploration of optimal preparation strategy of Chenpi (pericarps of Citrus reticulata Blanco) flavouring essence with great application potential in sugar and salt-reduced foods. Food Res. Int. 2024, 175, 113669. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, L.; Hu, Y.; Zhu, Z.; Zhuang, C.; Zhao, Y.; Zhong, Y. The preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. Int. J. Biol. Macromol. 2020, 151, 278–285. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Wang, D.; Zhong, Y.; Deng, Y. Combination of chitosan coating and heat shock treatments to maintain postharvest quality and alleviate cracking of Akebia trifoliate fruit during cold storage. Food Chem. 2022, 394, 133330. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Y.; Li, Y.; Jiang, Y.; Hu, X.; Yi, J. Chemometric Classification of Apple Cultivars Based on Physicochemical Properties: Raw Material Selection for Processing Applications. Foods 2023, 12, 3095. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, C.; Zhang, Y.; Jiang, A.; Hu, W. Aqueous ozone treatment inhibited degradation of cellwall polysaccharides in fresh-cut apple during cold storage. Innov. Food Sci. Emerg. Technol. 2021, 67, 102550. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Technol. 2020, 59, 102256. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples. Coatings 2019, 9, 503. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.E.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT 2019, 100, 341–347. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Castillejo, N.; Artés-Hernández, F. Effect of fresh–cut apples fortification with lycopene microspheres, revalorized from tomato by-products, during shelf life. Postharvest Biol. Technol. 2019, 156, 110925. [Google Scholar] [CrossRef]
- Wong, J.X.; Ramli, S.; Desa, S.; Chen, S.N. Use of Centella asiatica extract in reducing microbial contamination and browning effect in fresh cut fruits and vegetables during storage: A potential alternative of synthetic preservatives. LWT 2021, 151, 112229. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, P. Characterization of ester odorants of apple juice by gas chromatography-olfactometry, quantitative measurements, odour threshold, aroma intensity and electronic nose. Food Res. Int. 2019, 120, 92–101. [Google Scholar] [CrossRef]
- Laksana, A.J.; Kim, J.-H.; Ahn, J.-H.; Kim, J.-Y. Volatile Compounds and Quality Characteristics of Fresh-Cut Apples and Mixed Fruits Coated with Ascorbic Acid during Cold Storage. Agriculture 2024, 14, 474. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Li, G.; Du, Y.; Yi, J.; Hu, X.; Jiang, Y. Enhancing Fresh-Cut Apple Preservation: Impact of Slightly Acidic Electrolyzed Water and Chitosan–Apple Essence Microencapsulation Coating on Browning and Flavor. Foods 2024, 13, 1585. https://doi.org/10.3390/foods13101585
Luo Z, Li G, Du Y, Yi J, Hu X, Jiang Y. Enhancing Fresh-Cut Apple Preservation: Impact of Slightly Acidic Electrolyzed Water and Chitosan–Apple Essence Microencapsulation Coating on Browning and Flavor. Foods. 2024; 13(10):1585. https://doi.org/10.3390/foods13101585
Chicago/Turabian StyleLuo, Zhenyu, Guijing Li, Yanlin Du, Junjie Yi, Xiaosong Hu, and Yongli Jiang. 2024. "Enhancing Fresh-Cut Apple Preservation: Impact of Slightly Acidic Electrolyzed Water and Chitosan–Apple Essence Microencapsulation Coating on Browning and Flavor" Foods 13, no. 10: 1585. https://doi.org/10.3390/foods13101585




