Preferential Binding of Polyphenols in Blackcurrant Extracts with Milk Proteins and the Effects on the Bioaccessibility and Antioxidant Activity of Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Polyphenol Stock Solutions
2.2.2. Casein and Whey Protein Solutions
2.2.3. Milk Protein–Polyphenol Systems
2.3. Loading Efficiency Studies
2.4. Solvent-Based Extraction Procedure for HPLC
2.5. HPLC Quantification of Polyphenols
2.6. Fourier-Transform Infrared (FT-IR) Spectra
2.7. In Vitro Bioaccessibility in the Gastric Phase
2.8. Total Phenolic Content Measurement
2.9. The ABTS (2,2′-Amino-Di (-Ethyl-Benzothiazoline Sulphonic Acid-6) Ammonium Salt) Antioxidant Assay
2.10. Statistical Analyses
3. Results and Discussion
3.1. Total Phenolic Content and Polyphenol Profile of Blackcurrant Extract and Milk Protein-Based Samples
3.2. Preferential Binding and the Competition of Polyphenols for Binding with Milk Proteins
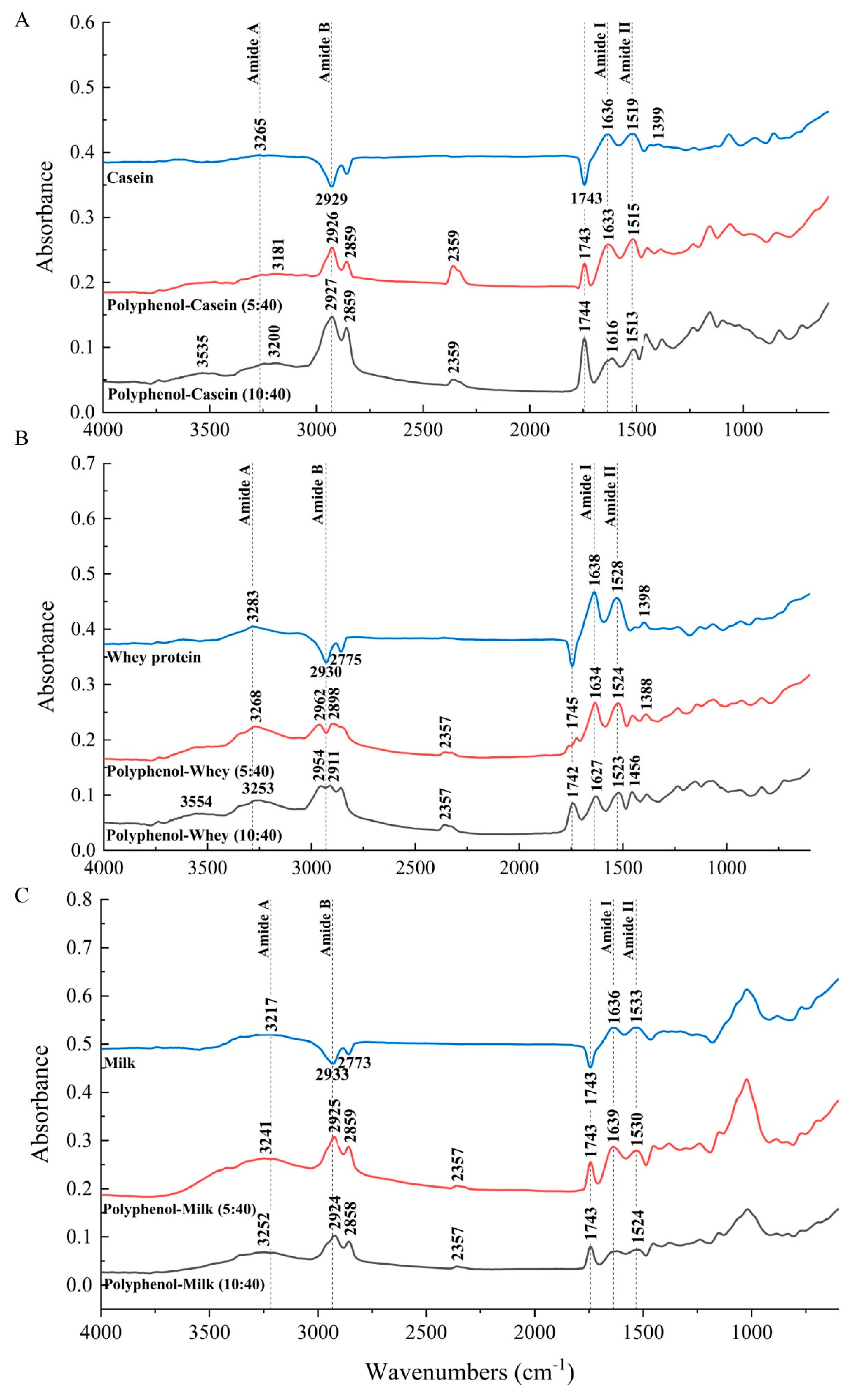
3.3. FT-IR Spectra of Polyphenol-Milk Protein System
3.4. Bioaccessibility and Antioxidant Activity of the Polyphenols Interacting with Milk Proteins in the Gastric Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Wu, Y.; Wang, D.; Wei, Y.; Wu, J.; Ji, B. Stability and absorption of anthocyanins from blueberries subjected to a simulated digestion process. Int. J. Food Sci. Nutr. 2014, 65, 440–448. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Ayyash, M.; Tarique, M.; Alaryani, M.; Al-Sbiei, A.; Masad, R.; Al-Saafeen, B.; Fernandez-Cabezudo, M.; al-Ramadi, B.; Kizhakkayil, J.; Kamal-Eldin, A. Bioactive properties and untargeted metabolomics analysis of bioaccessible fractions of non-fermented and fermented date fruit pomace by novel yeast isolates. Food Chem. 2022, 396, 133666. [Google Scholar] [CrossRef]
- Mao, T.; Wescombe, P.; Mohan, M.S. Predominance of non-covalent interactions of polyphenols with milk proteins and their health promoting properties. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef]
- Walton, M.C.; Hendriks, W.H.; Broomfield, A.M.; McGhie, T.K. Viscous food matrix influences absorption and excretion but not metabolism of blackcurrant anthocyanins in rats. J. Food Sci. 2009, 74, H22–H29. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hui, X.; Stipkovits, L.; Rachman, A.; Tu, J.; Brennan, M.A.; Brennan, C.S. Whey protein-blackcurrant concentrate particles obtained by spray-drying and freeze-drying for delivering structural and health benefits of cookies. Innov. Food Sci. Emerg. Technol. 2021, 68, 102606. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Interactions between Blackcurrant Polyphenols and Food Macronutrients in Model Systems: In Vitro Digestion Studies. Foods 2021, 10, 847. [Google Scholar] [CrossRef]
- Jaworska, G.; Sady, M.; Grega, T.; Bernaś, E.; Pogoń, K. Qualitative comparison of blackcurrant and blackcurrant–whey beverages. Food Sci. Technol. Int. 2011, 17, 331–341. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Matumoto-Pintro, P.T.; Bazinet, L.; Couillard, C.; Britten, M. Effect of commercial grape extracts on the cheese-making properties of milk. J. Dairy Sci. 2015, 98, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef]
- Cebeci, F.; Şahin-Yeşilçubuk, N. The matrix effect of blueberry, oat meal and milk on polyphenols, antioxidant activity and potential bioavailability. Int. J. Food Sci. Nutr. 2014, 65, 69–78. [Google Scholar] [CrossRef]
- Grimbleby, F.H.; Ntailianas, H.A. Binding of Trichloroacetic Acid by Protein. Nature 1961, 189, 835–836. [Google Scholar] [CrossRef]
- Kaur, G.; Alam, M.S.; Jabbar, Z.; Javed, K.; Athar, M. Evaluation of antioxidant activity of Cassia siamea flowers. J. Ethnopharmacol. 2006, 108, 340–348. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Archaina, D.; Vasile, F.; Jiménez-Guzmán, J.; Alamilla-Beltrán, L.; Schebor, C. Physical and functional properties of roselle (Hibiscus sabdariffa L.) extract spray dried with maltodextrin-gum arabic mixtures. J. Food Process. Preserv. 2019, 43, e14065. [Google Scholar] [CrossRef]
- Wang, L.; Brennan, M.A.; Guan, W.; Liu, J.; Zhao, H.; Brennan, C.S. Edible mushrooms dietary fibre and antioxidants: Effects on glycaemic load manipulation and their correlations pre-and post-simulated in vitro digestion. Food Chem. 2021, 351, 129320. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z.; Matusevicius, P.; Kołodziejczyk, K. Polyphenol-rich extract from blackcurrant pomace attenuates the intestinal tract and serum lipid changes induced by a high-fat diet in rabbits. Eur. J. Nutr. 2014, 53, 1603–1613. [Google Scholar] [CrossRef]
- Rehan, F.; Ahemad, N.; Gupta, M. Casein nanomicelle as an emerging biomaterial—A comprehensive review. Colloids Surf. B Biointerfaces 2019, 179, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Eskin, N.A.M.; Goff, H.D. Chapter 4—Milk. In Biochemistry of Foods, 3rd ed.; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 187–214. [Google Scholar]
- Liu, C.; He, W.; Chen, S.; Chen, J.; Zeng, M.; Qin, F.; He, Z. Interactions of digestive enzymes and milk proteins with tea catechins at gastric and intestinal pH. Int. J. Food Sci. Technol. 2017, 52, 247–257. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Zhang, H.; Yu, D.; Jin, S.; Ren, F. Interaction of chlorogenic acid with milk proteins analyzed by spectroscopic and modeling methods. Spectrosc. Lett. 2016, 49, 44–50. [Google Scholar] [CrossRef]
- Poonia, A. Potential of milk proteins as nanoencapsulation materials in food industry. Nanosci. Food Agric. 2017, 5, 139–168. [Google Scholar]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Milenkova, S.; Pilicheva, B.; Uzunova, Y.; Yovcheva, T.; Marudova, M. Casein Microgels as Benzydamine Hydrochloride Carriers for Prolonged Release. Materials 2022, 15, 1333. [Google Scholar] [CrossRef]
- Perlstein, H.; Bavli, Y.; Turovsky, T.; Rubinstein, A.; Danino, D.; Stepensky, D.; Barenholz, Y. Beta-casein nanocarriers of celecoxib for improved oral bioavailability. Eur. J. Nanomed. 2014, 6, 217–226. [Google Scholar] [CrossRef]
- Rehan, F.; Emranul Karim, M.; Ahemad, N.; Farooq Shaikh, M.; Gupta, M.; Gan, S.H.; Chowdhury, E.H. A comparative evaluation of anti-tumor activity following oral and intravenous delivery of doxorubicin in a xenograft model of breast tumor. J. Pharm. Investig. 2022, 52, 787–804. [Google Scholar] [CrossRef]
- Turovsky, T.; Portnaya, I.; Kesselman, E.; Ionita-Abutbul, I.; Dan, N.; Danino, D. Effect of temperature and loading on the structure of β-casein/ibuprofen assemblies. J. Colloid Interface Sci. 2015, 449, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Raj, J.; Uppuluri, K.B. Metformin Loaded Casein Micelles for Sustained Delivery: Formulation, Characterization and in-Vitro Evaluation. Biomed. Pharmacol. J. 2015, 8, 83–89. [Google Scholar] [CrossRef]
- Rehan, F.; Ahemad, N.; Islam, R.A.; Gupta, M.; Gan, S.H.; Chowdhury, E.H. Optimization and formulation of nanostructured and self-assembled caseinate micelles for enhanced cytotoxic effects of paclitaxel on breast cancer cells. Pharmaceutics 2020, 12, 984. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Mohan, M.; Dunlap, J.; Harte, F. Effect of Ultra-High Pressure Homogenization on the Interaction Between Bovine Casein Micelles and Ritonavir. Pharm. Res. 2015, 32, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Rosenberg, D.; Livney, Y. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ϖ-3 polyunsaturated fatty acids. Food Hydrocoll. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P. Spray- or freeze-drying of casein micelles loaded with Vitamin D2: Studies on storage stability and in vitro digestibility. LWT 2018, 97, 87–93. [Google Scholar] [CrossRef]
- Mohan, M.S.; Jurat-Fuentes, J.L.; Harte, F. Binding of vitamin A by casein micelles in commercial skim milk. J. Dairy Sci. 2013, 96, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Bourassa, P.; Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Review on the loading efficacy of dietary tea polyphenols with milk proteins. Food Hydrocoll. 2018, 77, 322–328. [Google Scholar] [CrossRef]
- Onufriev, A.V.; Alexov, E. Protonation and pK changes in protein-ligand binding. Q. Rev. Biophys. 2013, 46, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Rahmelow, K.; Hübner, W. Secondary structure determination of proteins in aqueous solution by infrared spectroscopy: A comparison of multivariate data analysis methods. Anal. Biochem. 1996, 241, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Voinitchi, C.; Gaidau, C.; Capatana Tudorie, F.; Niculescu, M.; Stanca, M.; Alexe, C.-A. Collagen and Keratin Hydrolysates to Delay the Setting of Gypsum Plaster. Materials 2022, 15, 8817. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.N.; Jacobs, T.M.; Kuhlman, B. Boosting protein stability with the computational design of β-sheet surfaces. Protein Sci. 2016, 25, 702–710. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Gao, Y.; McClements, D.J. Food-grade covalent complexes and their application as nutraceutical delivery systems: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Li, D.; Tang, H.; Yu, D.; Wang, L.; Jiang, L. Effect of anionic polysaccharides on conformational changes and antioxidant properties of protein-polyphenol binary covalently-linked complexes. Process Biochem. 2020, 89, 89–97. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.-A. Interaction of milk α- and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Salah, M.; Mansour, M.; Zogona, D.; Xu, X. Nanoencapsulation of anthocyanins-loaded β-lactoglobulin nanoparticles: Characterization, stability, and bioavailability in vitro. Food Res. Int. 2020, 137, 109635. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Li, B.; Gong, E.; Shu, C.; Si, X.; Gao, N.; Zhang, W.; Cui, H.; Meng, X. Effects of α-casein and β-casein on the stability, antioxidant activity and bioaccessibility of blueberry anthocyanins with an in vitro simulated digestion. Food Chem. 2021, 334, 127526. [Google Scholar] [CrossRef] [PubMed]






| β-Sheet/% | Random Roil/% | α-Helix/% | β-Turn/% | |
|---|---|---|---|---|
| Casein | 46.43 ± 2.85 b | 8.08 ± 0.94 a | 29.21 ± 3.65 bc | 16.28 ± 1.74 b |
| Blackcurrant–Casein (5:40) | 40.98 ± 1.23 c | 5.58 ± 1.28 b | 25.29 ± 1.91 c | 28.14 ± 3.41 a |
| Blackcurrant–Casein (10:40) | 39.98 ± 1.7 c | 6.05 ± 1.59 b | 24.64 ± 1.91 c | 29.33 ± 3.16 a |
| Whey | 46.71 ± 1.36 b | 8.05 ± 0.65 a | 27.23 ± 0.56 c | 18.01 ± 0.41 b |
| Blackcurrant–Whey (5:40) | 49.37 ± 1.5 b | 7.44 ± 0.33 a | 27.3 ± 2.15 c | 15.88 ± 3.36 b |
| Blackcurrant–Whey (10:40) | 53.22 ± 2.54 a | 5.19 ± 0.89 b | 26.91 ± 2.82 c | 14.68 ± 4.04 b |
| Milk | 48.54 ± 1.71 b | 5.95 ± 1.02 b | 27.38 ± 0.46 bc | 18.14 ± 0.41 b |
| Blackcurrant–Milk (5:40) | 39.77 ± 1.14 c | 7.51 ± 0.15 a | 34.72 ± 3.55 a | 17.99 ± 4.41 b |
| Blackcurrant–Milk (10:40) | 40.99 ± 2 c | 6.2 ± 0.82 a | 35.15 ± 1.53 a | 17.65 ± 2.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, T.; Akshit, F.; Matiwalage, I.; Sasidharan, S.; Alvarez, C.M.; Wescombe, P.; Mohan, M.S. Preferential Binding of Polyphenols in Blackcurrant Extracts with Milk Proteins and the Effects on the Bioaccessibility and Antioxidant Activity of Polyphenols. Foods 2024, 13, 515. https://doi.org/10.3390/foods13040515
Mao T, Akshit F, Matiwalage I, Sasidharan S, Alvarez CM, Wescombe P, Mohan MS. Preferential Binding of Polyphenols in Blackcurrant Extracts with Milk Proteins and the Effects on the Bioaccessibility and Antioxidant Activity of Polyphenols. Foods. 2024; 13(4):515. https://doi.org/10.3390/foods13040515
Chicago/Turabian StyleMao, Ting, FNU Akshit, Iresha Matiwalage, Subha Sasidharan, Caren Meyn Alvarez, Philip Wescombe, and Maneesha S. Mohan. 2024. "Preferential Binding of Polyphenols in Blackcurrant Extracts with Milk Proteins and the Effects on the Bioaccessibility and Antioxidant Activity of Polyphenols" Foods 13, no. 4: 515. https://doi.org/10.3390/foods13040515





