Influence of Four Veterinary Antibiotics on Constructed Treatment Wetland Nitrogen Transformation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection for Microcosms
2.2. Microcosm Experimental Setup
2.2.1. Anaerobic Microcosms
2.2.2. Aerobic Microcosms
2.3. Mesocosm Experiment Setup
2.4. Analytical Methods
2.4.1. Microcosm Analytical Methods
2.4.2. Mesocosm Analytical Methods
2.4.3. Microbial Analytical Methods
2.4.4. Antibiotic Analytical Methods
2.5. NO3-N Removal Rates
2.6. Statistical Analyses
3. Results
3.1. Microcosm Experiments
3.1.1. Microcosm Experimental Conditions
3.1.2. Nitrification Potential Microcosm Experiments
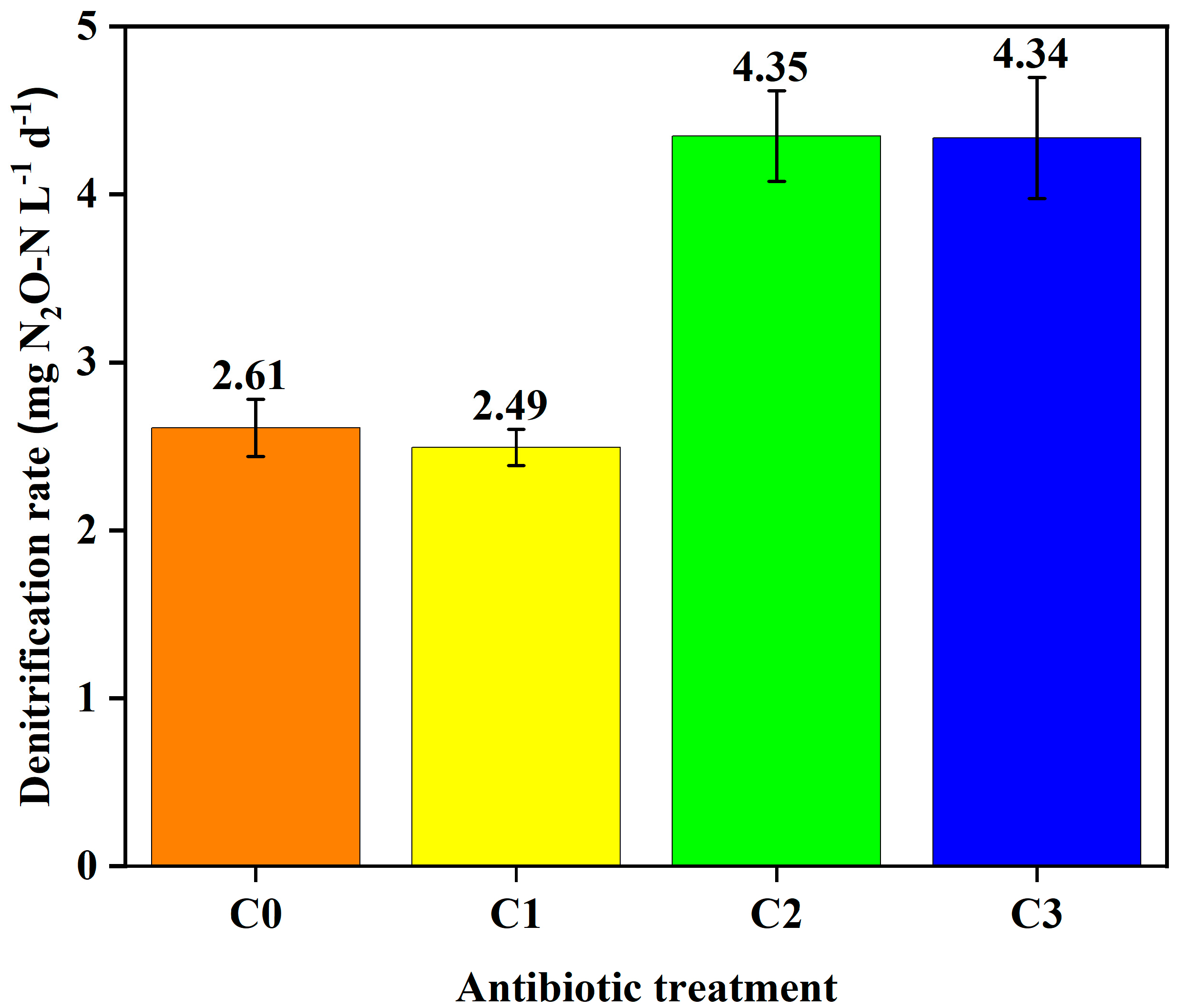
3.1.3. Denitrification Potential Microcosm Experiments
3.1.4. Microbial Community Variability
3.2. Mesocosm Experiment
3.2.1. NO3-N Removal following Exposure to Veterinary Antibiotics
3.2.2. Physiochemical Fluctuations
3.2.3. VA Recovery in Water
3.2.4. Physiochemical Relationships to Antibiotic Concentrations
3.2.5. Plant Uptake of Veterinary Antibiotics
4. Discussion
4.1. Microcosm Nitrification Rates
4.2. Microcosm Denitrification Rates
4.3. Microcosm Microbial Community
4.4. Mesocosm Physiochemical Parameters
4.5. Mesocosm Nitrate Reduction
4.6. Mesocosm Plant Biomass VA Uptake/Adsorption
4.7. Agrochemical Mixture Studies
5. Conclusions/Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VA | Veterinary Antibiotic |
| CAFO | Confined Animal Feeding Operation |
| FTW | Floating Treatment Wetland |
| USDA | United States Department of Agriculture |
| USMARC | United States Meat Animal Research Center |
| USGS | United States Geological Survey |
| DOC | Dissolved Organic Carbon |
| TDN | Total Dissolved Nitrogen |
| ORP | Oxidation Reduction Potential |
| DO | Dissolved Oxygen |
| HDPE | High-Density Polyethylene |
| LC-MS/MS | Liquid Chromatography–Mass Spectrometry/Mass Spectrometry |
References
- Exner, M.E.; Hirsh, A.J.; Spalding, R.F. Nebraska’s groundwater legacy: Nitrate contamination beneath irrigated cropland. Water Resour. Res. 2014, 50, 4474–4489. [Google Scholar] [CrossRef]
- Nolan, B.T.; Hitt, K.J. Vulnerability of shallow groundwater and drinking-water wells to nitrate in the united states. Environ. Sci. Technol. 2006, 40, 7834–7840. [Google Scholar] [CrossRef] [PubMed]
- EPA. National Primary Drinking Water Regulations [WWW Document]. 2009. Available online: https://www.epa.gov/sites/default/files/2016-06/documents/npwdr_complete_table.pdf (accessed on 1 June 2021).
- Mekonnen, M.M.; Hoekstra, A.Y. Global gray water footprint and water pollution levels related to anthropogenic nitrogen loads to fresh water. Environ. Sci. Technol. 2015, 49, 12860–12868. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.; Cedergreen, N.; Hayes, T.B.; Hansen, M. Nitrate: An environmental endocrine disruptor? A review of evidence and research needs. Environ. Sci. Technol. 2018, 52, 3869–3887. [Google Scholar] [CrossRef] [PubMed]
- Kross, B.C.; Ayebo, A.D.; Fuortes, L.J. Methemoglobinemia: Nitrate toxicity in rural America. Am. Fam. Physician 1992, 46, 183–188. [Google Scholar] [PubMed]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Winchester, P.D.; Huskins, J.; Ying, J. Agrichemicals in surface water and birth defects in the United States. Acta Paediatr. 2009, 98, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking Water Nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Causse, J.; Baurès, E.; Mery, Y.; Jung, A.-V.; Thomas, O. Variability of N export in water: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2245–2281. [Google Scholar] [CrossRef]
- Paredes, I.; Otero, N.; Soler, A.; Green, A.J.; Soto, D.X. Agricultural and urban delivered nitrate pollution input to Mediterranean temporary freshwaters. Agric. Ecosyst. Environ. 2020, 294, 106859. [Google Scholar] [CrossRef]
- Torres-Martínez, J.A.; Mora, A.; Mahlknecht, J.; Daesslé, L.W.; Cervantes-Avilés, P.A.; Ledesma-Ruiz, R. Estimation of nitrate pollution sources and transformations in groundwater of an intensive livestock-agricultural area (Comarca Lagunera), combining major ions, stable isotopes and MixSIAR model. Environ. Pollut. 2020, 269, 115445. [Google Scholar] [CrossRef] [PubMed]
- Bager, F. DANMAP: Monitoring antimicrobial resistance in Denmark. Int. J. Antimicrob. Agents 2000, 14, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Deckert, A.; Gow, S.; Rosengren, L.; Léger, D.; Avery, B.; Daignault, D.; Dutil, L.; Reid-Smith, R.; Irwin, R. Canadian integrated program for antimicrobial resistance surveillance (CIPARS) farm program: Results from finisher pig surveillance. Zoonoses Public Health 2010, 57, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Williams-Nguyen, J.; Sallach, J.B.; Bartelt-Hunt, S.; Boxall, A.B.; Durso, L.M.; McLain, J.E.; Singer, R.S.; Snow, D.D.; Zilles, J.L. Antibiotics and antibiotic resistance in agroecosystems: State of the science. J. Environ. Qual. 2016, 45, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Trejo, B.; Russell, M.; Bartelt-Hunt, S.; Beni, N.N.; Snow, D.D.; Messer, T.L. Occurrence and persistence of antibiotics administered to cattle in a newly established feedlot. J. Environ. Qual. 2023, 52, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Okano, K.; Zhang, C.; Zhang, Z.; Lei, Z.; Feng, C.; Utsumi, M.; Ihara, I.; Maseda, H.; Shimizu, K. Effects of levofloxacin exposure on sequencing batch reactor (SBR) behavior and microbial community changes. Sci. Total Environ. 2019, 672, 227–238. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Ong, S.K.; Moorman, T.B. Dissipation of atrazine, enrofloxacin, and sulfamethazine in wood chip bioreactors and impact on denitrification. J. Environ. Qual. 2011, 40, 1816–1823. [Google Scholar] [CrossRef]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.-M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Whitacre, D.M.; Monteiro, S.C.; Boxall, A.B. Occurrence and fate of human pharmaceuticals in the environment. Rev. Environ. Contam. Toxicol. 2010, 202, 53–154. [Google Scholar] [CrossRef] [PubMed]
- Roose-Amsaleg, C.; Laverman, A.M. Do antibiotics have environmental side-effects? Impact of synthetic antibiotics on biogeochemical processes. Environ. Sci. Pollut. Res. 2015, 23, 4000–4012. [Google Scholar] [CrossRef]
- Xia, K.; Bhandari, A.; Das, K.; Pillar, G. Occurrence and fate of pharmaceuticals and personal care products (PPCPs) in biosolids. J. Environ. Qual. 2005, 34, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2020, 753, 141975. [Google Scholar] [CrossRef]
- Jaimes-Correa, J.C.; Snow, D.D.; Bartelt-Hunt, S.L. Seasonal occurrence of antibiotics and a beta agonist in an agriculturally-intensive watershed. Environ. Pollut. 2015, 205, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.D.; Cassada, D.A.; Monson, S.J.; Zhu, J.; Spalding, R.F. Tetracycline and Macrolide Antibiotics: Trace Analysis in Water and Wastewater Using Solid Phase Extraction and Liquid Chromatography—Tandem Mass Spectrometry. ACS Symp. Ser. 2003, 850, 161–174. [Google Scholar]
- Zhang, D.; Li, X.; Wang, M.; Xie, W. Occurrence and distribution of poly-and perfluoroalkyl substances (PFASs) in a surface flow constructed wetland. Ecol. Eng. 2021, 169. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Parker, D.B.; Snow, D.D.; Zhou, Z.; Li, X. Occurrence of antimicrobials and antimicrobial resistance genes in beef cattle storage ponds and swine treatment lagoons. Sci. Total Environ. 2013, 463–464, 631–638. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Huang, Y.; Wu, L.; Liu, X.; Luo, Y. Residues and risks of veterinary antibiotics in protected vegetable soils following application of different manures. Chemosphere 2016, 152, 229–237. [Google Scholar] [CrossRef]
- Bassil, R.J.; Bashour, I.I.; Sleiman, F.T.; Abou-Jawdeh, Y.A. Antibiotic uptake by plants from manure-amended soils. J. Environ. Sci. Health Part B 2013, 48, 570–574. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.-W.; Chang, H.-Q.; Li, Z.-J.; Xue, J.-M. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Caron, G.; Suffet, I.; Belton, T. Effect of dissolved organic carbon on the environmental distribution of nonpolar organic compounds. Chemosphere 1985, 14, 993–1000. [Google Scholar] [CrossRef]
- Ardón, M.; Morse, J.L.; Doyle, M.W.; Bernhardt, E.S. The Water quality consequences of restoring wetland hydrology to a large agricultural watershed in the southeastern coastal plain. Ecosystems 2010, 13, 1060–1078. [Google Scholar] [CrossRef]
- Bachand, P.A.; Horne, A.J. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol. Eng. 1999, 14, 17–32. [Google Scholar] [CrossRef]
- Chescheir, G.M.; Gilliam, J.W.; Skaggs, R.W.; Broadhead, R.G. Nutrient and sediment removal in forested wetlands receiving pumped agricultural drainage water. Wetlands 1991, 11, 87–103. [Google Scholar] [CrossRef]
- Karpuzcu, M.E.; Stringfellow, W.T. Kinetics of nitrate removal in wetlands receiving agricultural drainage. Ecol. Eng. 2012, 42, 295–303. [Google Scholar] [CrossRef]
- Zhu, J.; Snow, D.D.; Cassada, D.A.; Monson, S.J.; Spalding, R.F. Analysis of oxytetracycline, tetracycline, and chlortetracycline in water using solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2001, 928, 177–186. [Google Scholar] [CrossRef]
- Jamal, M.; Shareef, M.; Sajid, S. Lincomycin and tetracycline resistance in poultry. Review. Matrix Sci. Pharma 2017, 1, 33–38. [Google Scholar] [CrossRef]
- Dungan, R.S.; Snow, D.D.; Bjorneberg, D.L. Occurrence of antibiotics in an agricultural watershed in south-central Idaho. J. Environ. Qual. 2017, 46, 1455–1461. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine, 5th ed.; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Bartelt-Hunt, S.; Snow, D.D.; Damon-Powell, T.; Miesbach, D. Occurrence of steroid hormones and antibiotics in shallow groundwater impacted by livestock waste control facilities. J. Contam. Hydrol. 2011, 123, 94–103. [Google Scholar] [CrossRef]
- Bailey, E.; Brooks, A.G.F. Determination of methylmercury in biological samples by capillary gas chromatography with electron capture detection. Microchim. Acta 1992, 109, 121–123. [Google Scholar] [CrossRef]
- Keilhauer, M.G.; Messer, T.L.; Mittelstet, A.R.; Franti, T.G.; Corman, J. Nitrate removal by floating treatment wetlands amended with spent coffee: A mesocosm-scale evaluation. Trans. ASABE 2019, 62, 1619–1630. [Google Scholar] [CrossRef]
- Messer, T.L.; Burchell, M.R.; Birgand, F.; Broome, S.W.; Chescheir, G. Nitrate removal potential of restored wetlands loaded with agricultural drainage water: A mesocosm scale experimental approach. Ecol. Eng. 2017, 106, 541–554. [Google Scholar] [CrossRef]
- Messer, T.L.; Burchell, M.R.; Böhlke, J.; Tobias, C.R. Tracking the fate of nitrate through pulse-flow wetlands: A mesocosm scale 15N enrichment tracer study. Ecol. Eng. 2017, 106, 597–608. [Google Scholar] [CrossRef]
- Johnson, B.; Smith, E.; Ackerman, J.W.; Dye, S.; Polinsky, R.; Somerville, E.; Decker, C.; Little, D.; Pond, G.J.; D’Amico, E. Spatial convergence in major dissolved ion concentrations and implications of headwater mining for downstream water quality. J. Am. Water Resour. Assoc. 2019, 55, 247–258. [Google Scholar] [CrossRef]
- Repert, D.A.; Underwood, J.C.; Smith, R.L.; Song, B. Nitrogen cycling processes and microbial community composition in bed sediments in the Yukon River at Pilot Station. J. Geophys. Res. Biogeosci. 2014, 119, 2328–2344. [Google Scholar] [CrossRef]
- Aiken, G.R.; Gilmour, C.C.; Krabbenhoft, D.P.; Orem, W. Dissolved organic matter in the Florida Everglades: Implications for ecosystem restoration. Crit. Rev. Environ. Sci. Technol. 2011, 41, 217–248. [Google Scholar] [CrossRef]
- Smith, R.L.; Repert, D.A.; Barber, L.B.; LeBlanc, D.R. Long-term groundwater contamination after source removal—The role of sorbed carbon and nitrogen on the rate of reoxygenation of a treated-wastewater plume on Cape Cod, MA, USA. Chem. Geol. 2013, 337–338, 38–47. [Google Scholar] [CrossRef]
- Garside, C. A chemiluminescent technique for the determination of nanomolar concentrations of nitrate and nitrite in seawater. Mar. Chem. 1982, 11, 159–167. [Google Scholar] [CrossRef]
- Scheiner, D. Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res. 1976, 10, 31–36. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Supp. 1), 4516–4522. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Repert, D.A.; Reed, A.P.; Smith, R.L.; Bartelt-Hunt, S.; Messer, T.; Russell, M.; Snow, D.; Underwood, J.C. Biogeochemical and microbial data from microcosm experiments using wetland sediment to investigate the influence of antibiotics and a nitrification inhibitor in agricultural run-off on N-cycling processes, 2019–2020: U.S. Geological Survey data release, 2024.
- D’Alessio, M.; Durso, L.M.; Miller, D.N.; Woodbury, B.; Ray, C.; Snow, D.D. Environmental fate and microbial effects of monensin, lincomycin, and sulfamethazine residues in soil. Environ. Pollut. 2019, 246, 60–68. [Google Scholar] [CrossRef]
- Sallach, J.B.; Snow, D.; Hodges, L.; Li, X.; Bartelt-Hunt, S. Development and comparison of four methods for the extraction of antibiotics from a vegetative matrix. Environ. Toxicol. Chem. 2015, 35, 889–897. [Google Scholar] [CrossRef]
- Yi, X.; Bayen, S.; Kelly, B.C.; Li, X.; Zhou, Z. Improved detection of multiple environmental antibiotics through an optimized sample extraction strategy in liquid chromatography-mass spectrometry analysis. Anal. Bioanal. Chem. 2015, 407, 9071–9083. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, J.; Cheng, D.; Feng, Y.; Liu, Y.; Aly, H.M.; Li, Z. Uptake, translocation and distribution of three veterinary antibiotics in Zea mays L. Environ. Pollut. 2019, 250, 47–57. [Google Scholar] [CrossRef]
- Lindgren, J.K.; Messer, T.L.; Miller, D.N.; Snow, D.D.; Franti, T.G. Neonicotinoid pesticide and nitrate mixture removal and persistence in floating treatment wetlands. J. Environ. Qual. 2022, 51, 1246–1258. [Google Scholar] [CrossRef]
- Messer, T.L.; Miller, D.N.; Little, H.; Oathout, K. Nitrate-N removal rate variabilities in floating treatment wetland mesocosms with diverse planting and carbon amendment designs. Ecol. Eng. 2022, 174, 106444. [Google Scholar] [CrossRef]
- Messer, T.L.; Burchell, M.R.; Birgand, F. Comparison of four nitrate removal kinetic models in two distinct wetland restoration mesocosm systems. Water 2017, 9, 517. [Google Scholar] [CrossRef]
- Stream Solute Workshop. Concepts and methods for assessing solute dynamics in stream ecosystems. J. N. Am. Benthol. Soc. 1990, 9, 95–119. [Google Scholar] [CrossRef]
- Burghate, S.P.; Ingole, N.W. Biological denitrification—A review. J. Environ. Sci. Comput. Sci. Eng. Technol. 2014, 3, 9–28. [Google Scholar]
- Guan, A.; Qi, W.; Peng, Q.; Zhou, J.; Bai, Y.; Qu, J. Environmental heterogeneity determines the response patterns of microbially mediated N-reduction processes to sulfamethoxazole in river sediments. J. Hazard. Mater. 2021, 421, 126730. [Google Scholar] [CrossRef]
- Li, Z.-L.; Cheng, R.; Chen, F.; Lin, X.-Q.; Yao, X.-J.; Liang, B.; Huang, C.; Sun, K.; Wang, A.-J. Selective stress of antibiotics on microbial denitrification: Inhibitory effects, dynamics of microbial community structure and function. J. Hazard. Mater. 2020, 405, 124366. [Google Scholar] [CrossRef]
- Li, Z.-L.; Cao, D.; Bai, C.-H.; Yao, X.-J.; Lin, X.-Q.; Wang, S.-P.; Nan, J.; Liang, B.; Li, L.-W.; Sun, K.; et al. Influence on denitrifying community performance by the long-term exposure to sulfamethoxazole and chlortetracycline in the continuous-flow EGSB reactors. Environ. Res. 2021, 204, 111979. [Google Scholar] [CrossRef]
- DeVries, S.L.; Zhang, P. Antibiotics and the terrestrial nitrogen cycle: A review. Curr. Pollut. Rep. 2016, 2, 51–67. [Google Scholar] [CrossRef]
- Konopka, M.; Henry, H.A.; Marti, R.; Topp, E. Multi-year and short-term responses of soil ammonia-oxidizing prokaryotes to zinc bacitracin, monensin, and ivermectin, singly or in combination. Environ. Toxicol. Chem. 2014, 34, 618–625. [Google Scholar] [CrossRef]
- Toth, J.D.; Feng, Y.; Dou, Z. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol. Biochem. 2011, 43, 2470–2472. [Google Scholar] [CrossRef]
- Katipoglu-Yazan, T.; Pala-Ozkok, I.; Ubay-Cokgor, E.; Orhon, D. Acute impact of erythromycin and tetracycline on the kinetics of nitrification and organic carbon removal in mixed microbial culture. Bioresour. Technol. 2013, 144, 410–419. [Google Scholar] [CrossRef]
- DeVries, S.L.; Loving, M.; Li, X.; Zhang, P. The effect of ultralow-dose antibiotics exposure on soil nitrate and N2O flux. Sci. Rep. 2015, 5, 16818. [Google Scholar] [CrossRef]
- Philippot, L.; Hallin, S.; Schloter, M. Ecology of denitrifying prokaryotes in agricultural soil. Adv. Agron. 2007, 96, 249–305. [Google Scholar]
- Ma, H.; Ye, L.; Hu, H.; Zhang, L.; Ding, L.; Ren, H. Determination and variation of core bacterial community in a two-stage full-scale anaerobic reactor treating high-strength pharmaceutical wastewater. J. Microbiol. Biotechnol. 2017, 27, 1808–1819. [Google Scholar] [CrossRef]
- Bílková, Z.; Malá, J.; Hrich, K. Fate and behaviour of veterinary sulphonamides under denitrifying conditions. Sci. Total Environ. 2019, 695, 133824. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, X.; Jia, D.; Lyu, Y.; Hu, J.; Chen, Q.; Sun, W. Sediments alleviate the inhibition effects of antibiotics on denitrification: Functional gene, microbial community, and antibiotic resistance gene analysis. Sci. Total Environ. 2022, 804, 150092. [Google Scholar] [CrossRef]
- Zou, H.; He, J.-T.; He, B.-N.; Lao, T.-Y.; Liu, F.; Guan, X.-Y. Sensitivity assessment of denitrifying bacteria against typical antibiotics in groundwater. Environ. Sci. Process. Impacts 2019, 21, 1570–1579. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Ding, C.; He, J. Effect of antibiotics in the environment on microbial populations. Appl. Microbiol. Biotechnol. 2010, 87, 925–941. [Google Scholar] [CrossRef]
- Backhaus, T.; Porsbring, T.; Arrhenius, Å.; Brosche, S.; Johansson, P.; Blanck, H. Single-substance and mixture toxicity of five pharmaceuticals and personal care products to marine periphyton communities. Environ. Toxicol. Chem. 2011, 30, 2030–2040. [Google Scholar] [CrossRef]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Dawson; Murphy, K. The temperature dependency of biological denitrification. Water Res. 1972, 6, 71–83. [Google Scholar] [CrossRef]
- Patureau, D.; Bernet, N.; Delgenès, J.P.; Moletta, R. Effect of dissolved oxygen and carbon-nitrogen loads on denitrification by an aerobic consortium. Appl. Microbiol. Biotechnol. 2000, 54, 535–542. [Google Scholar] [CrossRef]
- Ahmad, M.; Vithanage, M.; Kim, K.; Cho, J.-S.; Lee, Y.H.; Joo, Y.K.; Lee, S.S.; Ok, Y.S. Inhibitory effect of veterinary antibiotics on denitrification in groundwater: A microcosm approach. Sci. World J. 2014, 2014, 879831. [Google Scholar] [CrossRef]
- Hou, L.; Yin, G.; Liu, M.; Zhou, J.; Zheng, Y.; Gao, J.; Zong, H.; Yang, Y.; Gao, L.; Tong, C. Effects of sulfamethazine on denitrification and the associated N2O release in estuarine and coastal sediments. Environ. Sci. Technol. 2014, 49, 326–333. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef]
- Huynh, K.; Reinhold, D. Uptake, translocation, and metabolism of sulfamethazine by Arabidopsis thaliana: Distinguishing between phytometabolites and abiotic transformation products in the media. Int. J. Phytoremediation 2019, 22, 412–419. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, Z.; Zhang, L.; Gong, C.; Yan, C. Removal of sulfonamides from water by wetland plants: Performance, microbial response and mechanism. Sci. Total Environ. 2024, 916, 170181. [Google Scholar] [CrossRef]
- Batchelder, A.R. Chlortetracycline and oxytetracycline effects on plant growth and development in soil systems. J. Environ. Qual. 1982, 11, 675–678. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic uptake by plants from soil fertilized with animal manure. J. Environ. Qual. 2005, 34, 2082–2085. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599-600, 500–512. [Google Scholar] [CrossRef]
- Xu, H.; Lu, G.; Xue, C. Effects of sulfamethoxazole and 2-ethylhexyl-4-methoxycinnamate on the dissimilatory nitrate reduction processes and N2O release in sediments in the Yarlung Zangbo river. Int. J. Environ. Res. Public Health 2020, 17, 1822. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.D.; Bernhardt, E. Are nitrogen and carbon cycle processes impacted by common stream antibiotics? A comparative assessment of single vs. mixture exposures. PLoS ONE 2022, 17, e0261714. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Liu, S.; Chen, Q.; Sun, W.; Zhong, H.; Hu, J.; Liang, E.; Ni, J. Response of microbial nitrogen transformation processes to antibiotic stress in a drinking water reservoir. Sci. Total Environ. 2021, 797, 149119. [Google Scholar] [CrossRef]







| Constituent | Concentration |
|---|---|
| Water | |
| Sodium, mg L−1 | 42.5 |
| Ammonium, mg N L−1 | 0.37 |
| Potassium, mg L−1 | 118 |
| Magnesium, mg L−1 | 10.8 |
| Calcium, mg L−1 | 31.2 |
| Chloride, mg L−1 | 81.6 |
| Nitrite, mg N L−1 | 0.035 |
| Nitrate, mg N L−1 | 4.89 |
| Phosphate, mg P L−1 | 4.34 |
| Sulfate, mg S L−1 | 18.7 |
| pH | 8.23 |
| Alkalinity, mg CaCO3 L−1 | 141 |
| DOC, mg C L−1 | 35.2 |
| TDN, mg N L−1 | 8.94 |
| Organic N, mg N L−1 | 3.64 |
| Reuse pit: Chlortetracycline, µg L−1 | <0.008 |
| Reuse pit: Lincomycin, µg L−1 | 0.76 |
| Reuse pit: Monensin, µg L−1 | 1.10 |
| Reuse pit: Sulfadimethoxine, µg L−1 | 0.48 |
| Sediment | |
| Total C, mg g−1 | 26.1 (1.28) a |
| Total N, mg g−1 | 2.98 (0.12) a |
| C/N, mg mg−1 | 8.77 |
| KCl Extr. NH4+, µg N gdw−1 | 33.4 (7.6) b |
| KCl Extr. NO3− + NO2−, µg N gdw−1 | 1.83 (1.53) b |
| KCl Extr. NO2−, µg N gdw−1 | 0.59 (0.1) b |
| Compound | Parent Ion (m/z) | Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | Retention Time (min) |
|---|---|---|---|---|---|
| Sulfamethazine-phenyl-13C8 | 285.10 | 123.95 | 30 | 25 | 11.04 |
| Doxycycline | 445.05 | 428.05 | 29 | 19 | 12.34 |
| Demeclocycline | 464.9 | 447.9 | 27 | 17 | 11.50 |
| Sulfachloropyridazine | 285.0 | 155.95 | 24 | 15 | 11.42 |
| Chlortetracycline (total) | 478.9 | 444.0 | 28 | 20 | 12.05 |
| Lincomycin | 407.0 | 126.0 | 38 | 25 | 8.94 |
| Monensin (ammonium adduct) | 688.1 | 635.15 | 22 | 17 | 17.01 |
| Monensin (sodium adduct) | 693.7 | 675.7 | 22 | 25 | 17.01 |
| Sulfadimethoxine | 311.05 | 155.95 | 28 | 20 | 12.55 |
| Sulfamethazine | 279.1 | 155.95 | 30 | 18 | 11.04 |
| Tylosin | 916.9 | 174.2 | 50 | 35 | 12.43 |
| Sample ID | Collection Date | Chlortetracycline (µg L−1) | Lincomycin (µg L−1) | Monensin (µg L−1) | Sulfadimethoxine (µg L−1) |
|---|---|---|---|---|---|
| Antibiotic Mix C0 Stock a | 12/18/2019 | <0.008 | 0.16 | <0.033 | <0.013 |
| Antibiotic Mix C1 Stock a | 12/18/2019 | <0.008 | 27.4 | 7.91 | 8.04 |
| Antibiotic Mix C2 Stock a | 12/18/2019 | 2.87 | 339 | 111 | 144 |
| Antibiotic Mix C3 Stock a | 12/18/2019 | 35.5 | 1682 | 324 | 631 |
| Preincubation Slurry b | 12/18/2019 | 0.559 (0.549) | 0.031 (0.022) | 0.133 (0.06) | 0.119 (0.087) |
| NF AB C0-TF c | 1/10/2020 | <0.008 | <0.027 | <0.033 | <0.013 |
| NF AB C1-TF c | 1/10/2020 | <0.008 | 0.586 (0.302) | <0.033 | 0.084 (0.042) |
| NF AB C2-TF c | 1/10/2020 | <0.008 | 6.30 (4.38) | <0.033 | 1.58 (0.594) |
| NF AB C3-TF c | 1/10/2020 | <0.008 | 57.7 (9.90) | 0.044 (0.009) | 3.91 (1.75) |
| DNF AB C0-TF d | 1/8/2020 | 0.081 (0.055) | 0.045 (0.017) | <0.033 | 0.000 |
| DNF AB C1-TF d | 1/8/2020 | <0.008 | 12.2 (0.643) | 2.22 (0.225) | 1.87 (0.04) |
| DNF AB C2-TF d | 1/8/2020 | 0.063 (0.018) | 139 (15) | 24.1 (10.7) | 29.5 (1.83) |
| DNF AB C3-TF d | 1/8/2020 | 0.462 (0.071) | 681 (55.3) | 182 (17.8) | 243 (25) |
| Treatment | DO (mg L−1) | Conductivity (μS cm−1) | ORP (mV) | Temperature (°C) | pH Range (min–max) | DOC (mg L−1) |
|---|---|---|---|---|---|---|
| Control | 3.9 ± 1.66 | 681.9 ± 10.27 | 90.33 ± 35.47 | 27.6 ± 1.23 | 6.55–7.29 | 4.29 ± 0.52 |
| Control Antibiotics | 1.76 ± 2.72 | 670.43 ± 11.9 | −90.9 ± 129.84 | 27.63 ± 1.37 | 6.57–7.31 | 79.51 ± 17.43 |
| FTW | 0.8 ± 0.16 | 873.71 ± 41.98 | 5.39 ± 70.96 | 26.23 ± 0.79 | 5.79–7.69 | 13.14 ± 1.86 |
| FTW + Antibiotics | 0.24 ± 0.2 | 882.38 ± 30.92 | −236.4 ± 97.99 | 25.6 ± 1.08 | 6.37–7.22 | 76.06 ± 6.2 |
| VA | Day | Control (mg) | Control-VA (mg) | FTW (mg) | FTW-VA (mg) |
|---|---|---|---|---|---|
| Chlortetracycline | 1 | <0.008 | <0.008 | <0.008 | 4.2 (±0.7) |
| 5 | <0.008 | <0.008 | <0.008 | 1.1 (±0.4) | |
| 10 | <0.008 | <0.008 | <0.008 | 0.9 (±0.2) | |
| Lincomycin | 1 | <0.027 | 106.2 (±28.7) | <0.027 | 208.8 (±39.0) |
| 5 | <0.027 | 105.5 (±29.7) | <0.027 | 254.3 (±47.6) | |
| 10 | <0.027 | 108.1 (±25.9) | <0.027 | 364.5 (±59.7) | |
| Monensin | 1 | <0.033 | 100.4 (±13.2) | <0.033 | 110.4 (±7.7) |
| 5 | <0.033 | 176.6 (±53.7) | <0.033 | 103.0 (±19.2) | |
| 10 | <0.033 | 347.8 (±18.2) | <0.033 | 250.4 (±76.4) | |
| Sulfamethazine | 1 | <0.013 | 265.1 (±12.4) | <0.013 | 185.2 (±20.4) |
| 5 | <0.013 | 108.2 (±53.9) | <0.013 | 287.0 (±49.1) | |
| 10 | <0.013 | 57.3 (±54.5) | <0.013 | 251.6 (±55.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, M.V.; Messer, T.L.; Repert, D.A.; Smith, R.L.; Bartelt-Hunt, S.; Snow, D.D.; Reed, A.P. Influence of Four Veterinary Antibiotics on Constructed Treatment Wetland Nitrogen Transformation. Toxics 2024, 12, 346. https://doi.org/10.3390/toxics12050346
Russell MV, Messer TL, Repert DA, Smith RL, Bartelt-Hunt S, Snow DD, Reed AP. Influence of Four Veterinary Antibiotics on Constructed Treatment Wetland Nitrogen Transformation. Toxics. 2024; 12(5):346. https://doi.org/10.3390/toxics12050346
Chicago/Turabian StyleRussell, Matthew V., Tiffany L. Messer, Deborah A. Repert, Richard L. Smith, Shannon Bartelt-Hunt, Daniel D. Snow, and Ariel P. Reed. 2024. "Influence of Four Veterinary Antibiotics on Constructed Treatment Wetland Nitrogen Transformation" Toxics 12, no. 5: 346. https://doi.org/10.3390/toxics12050346








