Isolation and Identification of Pathogens Causing Blue Mold of Lanzhou Lily during Postharvest Storage and Control of Disease and Mycotoxin Accumulation by Ozone Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Isolation and Purification of Isolates
2.2.2. Morphological Identification of Isolates
2.2.3. Molecular Biological Identification of Isolates
2.2.4. Pathogenicity Testing of Isolates
2.2.5. Control of Blue Mold of Lanzhou Lily by Ozone Treatment
2.2.6. Determination of Patulin
2.2.7. Statistical Analysis
3. Results
3.1. Development of Lanzhou Lily during Storage
3.2. Morphological Identification of Isolates
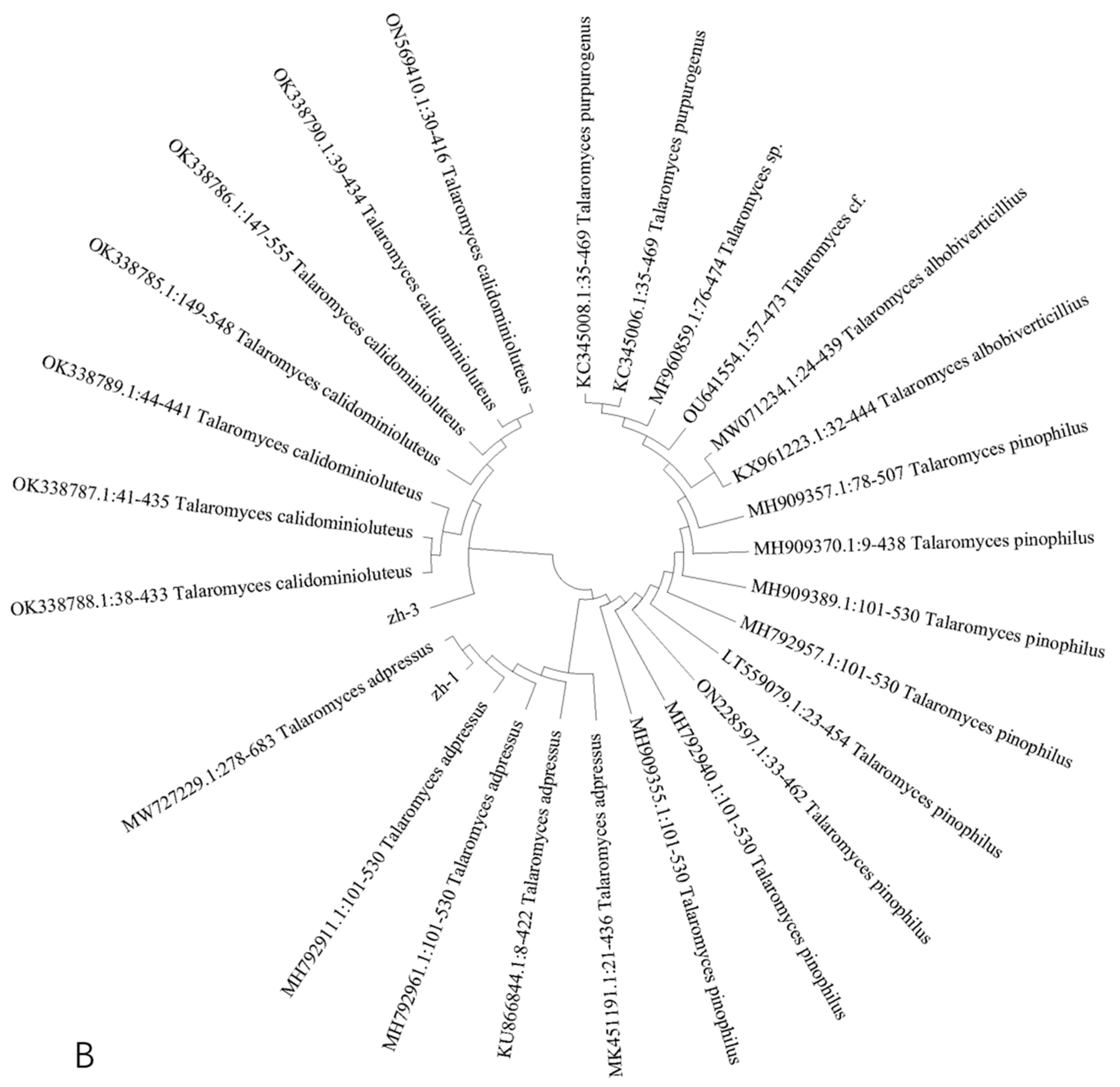
3.3. Molecular Biological Identification of Isolates
3.4. Pathogenicity Verification
3.5. Ozone Treatment for Disease Control
3.6. Effect of Ozone Treatment on Patulin Accumulation in the Lesion Tissue of Lanzhou Lily
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.X.; Chen, G.B.; Huang, D.J.; Wang, N.; Liao, W.B. The antioxidant defense system during Lanzhou lily scales storage is modulated by hydrogen sulfide. Horticulturae 2021, 7, 183. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Cheng, J.; Zhang, J.; da Silva, J.A.; Liu, X.; Duan, X.; Li, T.; Sun, H. Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor. BMC Plant Biol. 2014, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Zhang, M.; Chen, X.; Moe, T.S.; Du, Y.; Yang, F.; Xue, J.; Zhang, X. Isolation and characterization of plant growth-promoting endophytic bacteria Bacillus stratosphericus LW-03 from Lilium wardii. 3 Biotech 2020, 10, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.D. Disease control technology of Lanzhou lily during storage. CAJ Electron. Publ. House 2011, 21, 29–30. [Google Scholar] [CrossRef]
- Hu, Y.M.; Cao, C.X.; Lv, L.Z. Isolation and identification of pathogen of Lilium davidii var. unicolor scale air culture rot. Hubei Agric. Sci. 2022, 61, 84–87. [Google Scholar] [CrossRef]
- Wang, P.F. Research progress on the mechanism of action of agricultural biological agents and the control of lily diseases. MPB 2023, 1–21. Available online: https://kns.cnki.net/kcms2/detail/46.1068.s.20230712.1005.004.html (accessed on 22 July 2023).
- Ummat, V.; Singh, A.K.; Sidhu, G.K. Effect of aqueous ozone on quality and shelf life of shredded green bell pepper (Capsicum annuum). J. Food Process. Preserv. 2017, 42, 1–14. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Q.; Cao, Q.Z.; Zhao, Y.Q.; Liu, Q.; He, H.B.; Jia, G.X.; Zhang, D.M. Evaluation of resistance to Botrytis elliptica in Lilium hybrid cultivars. Plant Physiol. Bioch. 2018, 123, 392–399. [Google Scholar] [CrossRef]
- Tang, X.N. Investigation and identification of lily diseases in Jiangxi. Acta Agric. Jiangxi 1997, 9, 1–8. [Google Scholar] [CrossRef]
- Li, Y.F.; Xin, Q.; Zhang, Y.J.; Liang, M.X.; Zhao, G.; Jiang, D.Q.; Liu, X.H.; Zhang, H. Comparative metabolome analysis unravels a close association between dormancy release and metabolic alteration induced by low temperature in lily bulbs. Plant Cell Rep. 2022, 41, 1561–1572. [Google Scholar] [CrossRef]
- Yu, J.P.; Xu, S.J.; Liu, X.Y.; Li, T.; Zhang, D.H.; Teng, N.J.; Wu, Z. Starch degradation and sucrose accumulation of lily bulbs after cold storage. Int. J. Mol. Sci. 2022, 23, 4366. [Google Scholar] [CrossRef]
- Doorn, W.G.V.; Han, S.S. Postharvest quality of cut lily flowers. Postharvest Biol. Technol. 2011, 62, 1–6. [Google Scholar] [CrossRef]
- Glowacz, M.; Colgan, R.; Rees, D. The use of ozone to extend the shelf-life and maintain quality of fresh produce. J. Sci. Food Agric. 2015, 95, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Chrysargyris, A. Postharvest ozone application for the preservation of fruits and vegetables. Food Rev. Int. 2016, 33, 270–315. [Google Scholar] [CrossRef]
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A review on ozone-based treatments for fruit and vegetables preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Mei, K.; Asgar, A.; Aldersona, P.G.; Forneyd, C.F. Effect of different concentrations of ozone on physiological changes associated to gas exchange, fruit ripening, fruit surface quality and defence-related enzymes levels in papaya fruit during ambient storage. Sci. Hortic. 2014, 179, 163–169. [Google Scholar] [CrossRef]
- Lv, B.Y.; Yang, X.; Xue, H.L.; Shang, S.Q.; Nan, M.N.; Zhang, Y.; Liu, Z.G.; Bi, Y. Isolation of main pathogens causing postharvest disease in fresh Codonopsis pilosula during different storage stages and ozone control against disease and mycotoxin accumulation. J. Fungi 2023, 9, 146. [Google Scholar] [CrossRef]
- Xi, J.H.; Yang, D.Y.; Xue, H.L.; Liu, Z.G.; Bi, Y.; Zhang, Y. Isolation of the main pathogens causing postharvest disease in fresh Angelica sinensis during different storage stages and impacts of ozone treatment on disease development and mycotoxin production. Toxins 2023, 15, 154. [Google Scholar] [CrossRef]
- Aslam, R.; Alam, M.S.; Saeed, P.A. Sanitization potential of ozone and its role in postharvest quality management of fruits and vegetables. Food Eng. Rev. 2019, 12, 48–67. [Google Scholar] [CrossRef]
- Feng, L.N.; Wen, X.L.; Yang, W.J.; Wang, J.F.; Zhang, N.N.; Qi, H.X. Isolation and identification of Penicillium spp. causing Chinese chestnut seed rot during storage. Jiangsu Agric. Sci. 2021, 49, 164–168. [Google Scholar] [CrossRef]
- Navalkar, R.G.; Wiegeshau, E.H.; Smith, D.W. Relationship of mycoside content and colony morphology for group II and group III unclassified mycobacteria. J. Bacteriol. 1964, 88, 255–259. [Google Scholar] [CrossRef]
- Watrud, L.S.; Martin, K.; Donegan, K.K.; Stone, J.K.; Coleman, C.G. Comparison of taxonomic, colony morphotype and PCR-RFLP methods to characterize microfungal diversity. Mycologia 2017, 98, 384–392. [Google Scholar] [CrossRef]
- Wei, J.C. Fungal Identification Manual; Shanghai Scientific and Technical Publishers: Shanghai, China, 1979; p. 781. [Google Scholar]
- Zhu, R.Y.; Yu, T.; Guo, S.G.; Hu, H.; Zheng, X.; Petr, K. Effect of the yeast Rhodosporidium paludigenum on postharvest decay and patulin accumulation in apples and pears. J. Food Prot. 2015, 78, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hahm, S.S.; Oh, S.-Y.; Lee, E.-M.; Yu, S.-H. Identification of pathogens associated with bulb rot of lily during storage and effects of bulb disinfection on development of lily bulb rot. Res. Plant Dis. 2006, 21, 20–24. [Google Scholar] [CrossRef]
- Feng, C.P.; Li, Y.Q.; Na, L.J.; Gong, Y.W. The survey and identification of Lilium spp. diseases in Yuxi City. J. Fujian For. Sci. Technol. 2007, 34, 126–127. [Google Scholar] [CrossRef]
- Sadok, I.; Stachniuk, A.; Staniszewska, M. Developments in the monitoring of patulin in fruits using liquid chromatography: An overview. Food Anal. Methods 2018, 12, 76–93. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.; Marcone, M.F. Mitigation of patulin in fresh and processed foods and beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Qian, X.; Routledge, M.N.; Wu, X.Y.; Shi, Y.; Zhu, Q.; Zhang, H.Y. Metabonomics analysis of postharvest citrus response to Penicillium digitatum infection. LWT 2021, 152, 112371–112379. [Google Scholar] [CrossRef]
- Bacha, S.A.S.; Li, Y.; Nie, J.Y.; Xu, G.F.; Han, L.X.; Farooq, S. Comprehensive review on patulin and Alternaria toxins in fruit and derived products. Front. Plant Sci. 2023, 14, 1139757. [Google Scholar] [CrossRef]
- Yu, L.L.; Qiao, N.Z.; Zhao, J.X.; Zhang, H.; Tian, F.W.; Zhai, Q.X.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633–100711. [Google Scholar] [CrossRef]
- Gao, D.; Xu, X.J.; Mao, D.R. The research of the packaging technology of the ozone fruits and vegetable storage. Packag. Eng. 2004, 25, 138–141. [Google Scholar]
- Liew, C.L.; Prange, R.K. Effect of ozone and storage temperature on postharvest diseases and physiology of carrots (Daucus carota L.). J. Am. Soc. Hort. Sci. 1994, 119, 563–567. [Google Scholar] [CrossRef]
- Zapałowska, A.; Matłok, N.; Zardzewiały, M.; Piechowiak, T.; Balawejder, M. Effect of ozone treatment on the quality of sea buckthorn (Hippophae rhamnoides L.). Plants 2021, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Botondi, R.; Barone, M.; Grasso, C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]








| Gene | Gene | Primer Sequence |
|---|---|---|
| ITS | ITS1 | 5′-TCCGTAGGTGAACCTGCGG-3′ |
| ITS4 | 5′-TCCTCCGCTTATTGATATGC-3′ | |
| β-tubulin | Bt2a | 5′-GGTAACCAAATCGGTGCTGCTTTC-3′ |
| Bt2b | 5′-ACCCTCAGTGTAGTGACCCTTGGC-3′ |
| Disease Rating | Symptom |
|---|---|
| 0 | No disease |
| 1 | Scale disease area 0~25% |
| 2 | Scale disease area 25~50% |
| 3 | Scale disease area 50~75% |
| 4 | Scale disease area greater than 75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xi, J.; Liu, Z.; Chen, M.; Lu, Z.; Xue, H.; Bi, Y. Isolation and Identification of Pathogens Causing Blue Mold of Lanzhou Lily during Postharvest Storage and Control of Disease and Mycotoxin Accumulation by Ozone Treatment. J. Fungi 2023, 9, 1091. https://doi.org/10.3390/jof9111091
Zhang H, Xi J, Liu Z, Chen M, Lu Z, Xue H, Bi Y. Isolation and Identification of Pathogens Causing Blue Mold of Lanzhou Lily during Postharvest Storage and Control of Disease and Mycotoxin Accumulation by Ozone Treatment. Journal of Fungi. 2023; 9(11):1091. https://doi.org/10.3390/jof9111091
Chicago/Turabian StyleZhang, Hui, Jihui Xi, Zhiguang Liu, Minxuan Chen, Zhenhang Lu, Huali Xue, and Yang Bi. 2023. "Isolation and Identification of Pathogens Causing Blue Mold of Lanzhou Lily during Postharvest Storage and Control of Disease and Mycotoxin Accumulation by Ozone Treatment" Journal of Fungi 9, no. 11: 1091. https://doi.org/10.3390/jof9111091





