Structural Analyses of Polysaccharides Extracted from Cyanobacterial Extracellular Gels and Oriented Liquid Crystalline Microfiber Processing by Poly(vinyl alcohol)-Assisted Electrospinning
Abstract
:1. Introduction
2. Results and Discussion
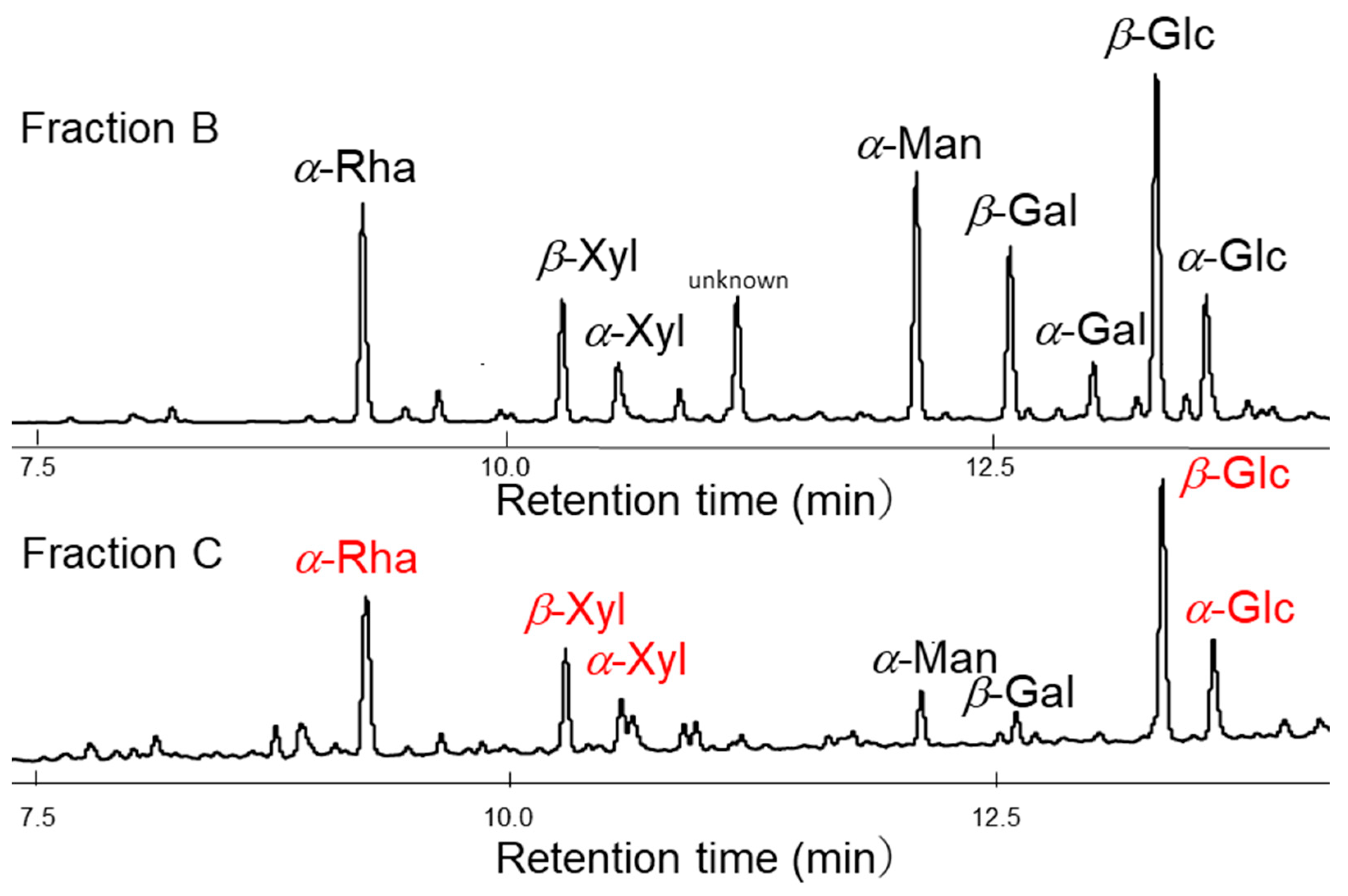
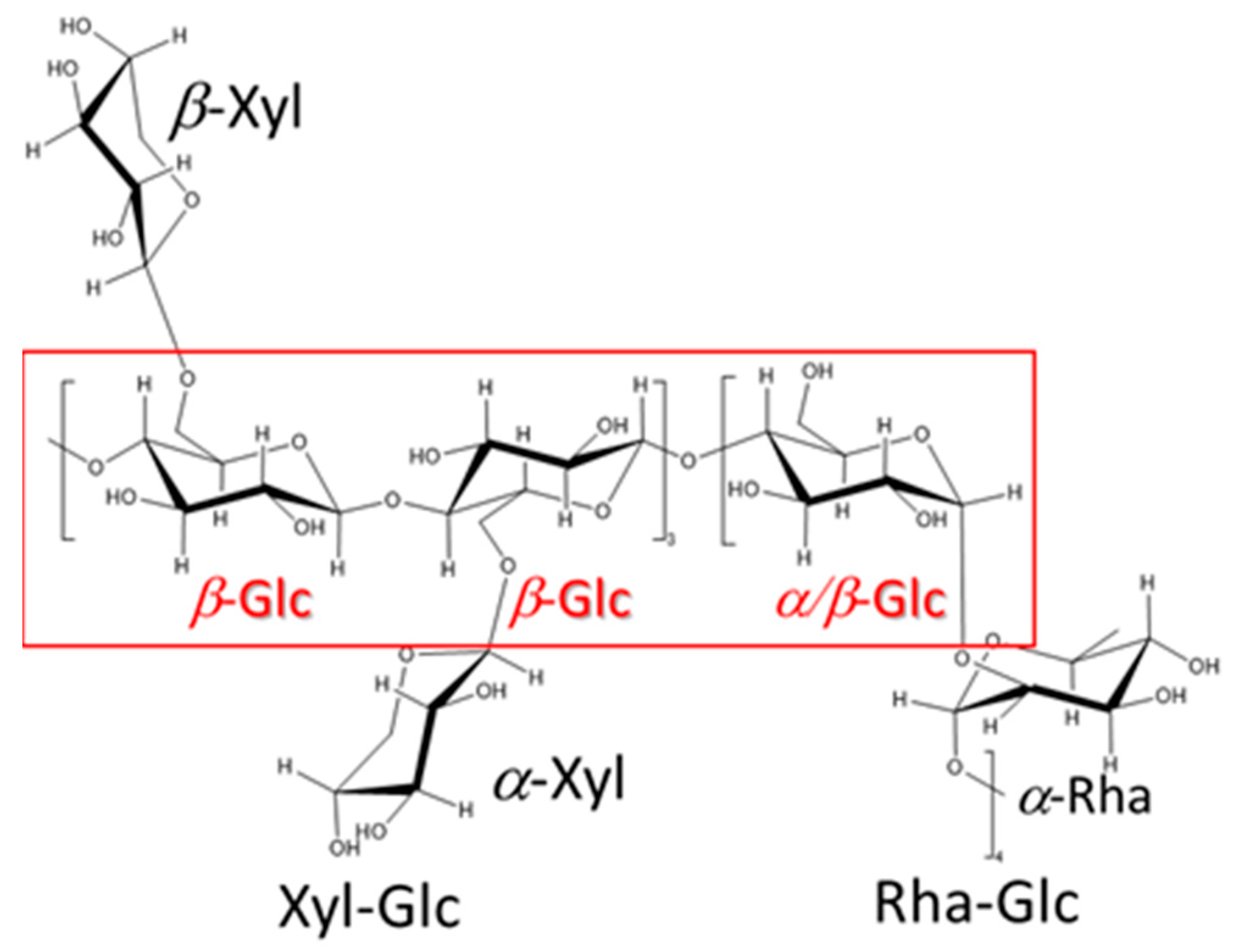
2.1. Structural Analyses
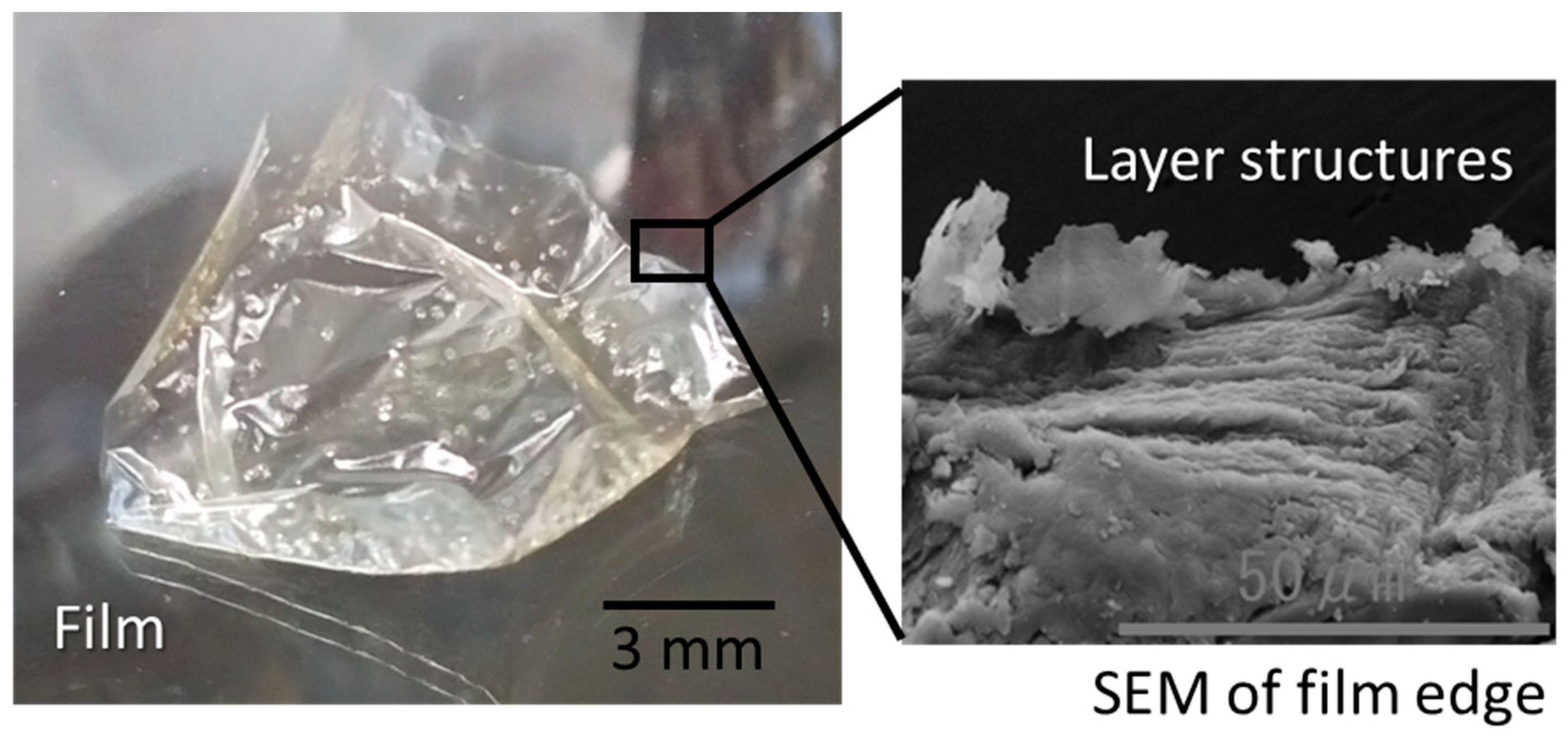
2.2. Electrospun Microfibers
2.3. Electrospun Microfibers
3. Materials and Methods
3.1. Material
3.2. Methods
3.2.1. Acid Hydrolyses and Fractionation
3.2.2. Monosaccharide Analyses
3.2.3. Electrospinning
3.2.4. Polarized Microscopy
3.2.5. Scanning Electron Microscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stortz, C.A.; Cerezo, A.S. Novel findings in carrageenans, agaroids and “hybrid” red seaweed galactans. Curr. Top. Phytochem. 2000, 4, 121. [Google Scholar]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Ueno, T.-M.; Okita, T. Absorption of color additives and settling volume in water of blue-green alga, ishikurage (Nostoc commune). Plant Foods Hum. Nutr. 1992, 42, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kabata, K.; Okamoto, C.; Sasada, N.; Ono, M.; Igoshi, K.; Kobayashi, H.; Masuoka, C.; Ito, Y. 日本固有種ラン藻・スイゼンジノリ(Aphanothece sacrum (Sur.) Okada)の培養および構成単糖と機能性の検索. Kyushu Tokai Daigaku Nogakubu Kiyo 2005, 24, 37. [Google Scholar]
- Watanabe, F.; Miyamoto, E.; Fujita, T.; Tanioka, Y.; Nakano, Y. Characterization of a Corrinoid Compound in the Edible (Blue-Green) Alga, Suizenji-nori. Biosci. Biotechnol. Biochem. 2006, 70, 3066–3068. [Google Scholar] [CrossRef] [PubMed]
- Ogura, F.; Hayashi, K.; Lee, J.B.; Kanekiya, K.; Hayashi, T. Evaluation of an Edible Blue-Green Alga, Aphanothece sacrum, for Its Inhibitory Effect on Replication of Herpes Simplex Virus Type 2 and Influenza Virus Type A. Biosci. Biotechnol. Biochem. 2010, 74, 1687–1690. [Google Scholar] [CrossRef]
- Fujishiro, T.; Ogawa, T.; Matsuoka, M.; Nagahama, K.; Takeshima, Y.; Hagiwara, H. Establishment of a Pure Culture of the Hitherto Uncultured Unicellular Cyanobacterium Aphanothece sacrum, and Phylogenetic Position of the Organism. Appl. Environ. Microbiol. 2004, 70, 3338. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Hana, S.; Matsumoto, M.; Yonejima, K.; Tansei, K.; Isogai, Y.; Igarashi, Y. Two new sacrolide-class oxylipins from the edible cyanobacterium Aphanothece sacrum. J. Antibiot. 2017, 70, 708–709. [Google Scholar] [CrossRef]
- Uchida, Y.; Maoka, T.; Palaga, T.; Honda, M.; Tode, C.; Shimizu, M.; Waditee-Sirisattha, R.; Kageyama, H. Identification of Desiccation Stress-Inducible Antioxidative and Antiglycative Ultraviolet-Absorbing Oxylipins, Saclipin A and Saclipin B, in an Edible Cyanobacterium Aphanothece sacrum. J. Agric. Food Chem. 2023, 71, 16137–16147. [Google Scholar] [CrossRef]
- Ren, S.; Gao, Y.; Wang, L.; Qiu, C.; Yang, L.; Li, L.; Xiao, Y.; Xiao, N.; Liao, L.; Zuo, Z.; et al. Sacran polysaccharide improves atopic dermatitis through inhibiting Th2 type immune response. Life Sci. 2022, 288, 120205. [Google Scholar] [CrossRef] [PubMed]
- Budpud, K.; Okeyoshi, K.; Kobayashi, S.; Okajima, M.K.; Kaneko, T. Super-Moisturizing Materials from Morphological Deformation of Suprapolysaccharides. Macromol. Rapid Commun. 2022, 43, 2200163. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yoo, S.-J.; Oh, D.-K.; Kweon, Y.-G.; Park, D.-W.; Lee, C.-H.; Gil, G.-H. Selection of a Streptococcus equimutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzym. Microb. Technol. 1996, 19, 440. [Google Scholar] [CrossRef]
- Vincent, J.F.V. From cellulose to cell. J. Exp. Biol. 1999, 202, 3263–3268. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Guille, M.M. Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif. Tissue Int. 1988, 42, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Badry, R.; El-Nahass, M.M.; Nada, N.; Elhaes, H.; Ibrahim, M.A. Structural and UV-blocking properties of carboxymethyl cellulose sodium/CuO nanocomposite films. Sci. Rep. 2023, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.; Suresh, C.H.; Thomas, T.; Thomas, T.J.; Pillai, C.K.S. Liquid crystalline phase behavior of high molecular weight DNA: A comparative study of the influence of metal ions of different size, charge and binding mode. Biomacromolecules 2008, 9, 1860–1869. [Google Scholar] [CrossRef]
- Budpud, K.; Okeyoshi, K.; Okajima, M.; Kaneko, T. Vapor-sensitive materials from polysaccharide fibers with self-assembling twisted microstructures. Small 2020, 16, 2001993. [Google Scholar] [CrossRef]
- Ali, M.A.; Singh, M.; Zhang, S.; Kaneko, D.; Okajima, M.K.; Kaneko, T. Metal-Assisted Injection Spinning of Ultra Strong Fibers from Megamolecular LC Polysaccharides. Polymers 2024, 16, 1099. [Google Scholar] [CrossRef]
- Yanaki, T.; Norisuye, T.; Teramoto, A. Cholesteric Mesophase in Aqueous Solutions of a Triple Helical Polysaccharide Scleroglucan. Polym. J. 1984, 16, 165–173. [Google Scholar] [CrossRef]
- Dong, X.M.; Gray, D.G. Effect of Counterions on Ordered Phase Formation in Suspensions of Charged Rodlike Cellulose Crystallites. Langmuir 1997, 13, 2404–2409. [Google Scholar] [CrossRef]
- Oertel, R.; Kulicke, W.M. Viscoelastic properties of liquid-crystals of aqueous biopolymer solutions. Rheol. Acta 1991, 30, 140–150. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular theory of liquid crystals. In Liquid Crystal Polymers I. Advances in Polymer Science; Platé, N.A., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 59, pp. 1–36. [Google Scholar] [CrossRef]
- De Gennes, P.G. Physics of Liquid Crystals; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Ngatu, N.R.; Okajima, M.K.; Yokogawa, M.; Ryoji Hirota, R.; Eitoku, M.; Muzembo, A.B.; Dumavibhat, N.; Takaishi, M.; Sano, S.; Kaneko, T.; et al. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2,4,6-trinitrochlorobenzene–induced allergic dermatitis in vivo. Ann. Allergy Asthma Immunol. 2012, 108, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Sagawa, Y.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H. Defensive Effects of a Unique Polysaccharide, Sacran, to Protect Keratinocytes against Extracellular Stimuli and Its Possible Mechanism of Action. Biol. Pharm. Bull. 2018, 41, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Sagawa, Y.; Sasano, K.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H. Protective Effects of Sacran, a Natural Polysaccharide, Against Adverse Effects on the Skin Induced by Tobacco Smoke. J. Cosmet. Sci. 2019, 70, 17–31. [Google Scholar]
- Motoyama, K.; Tanida, Y.; Sakai, A.; Higashi, T.; Kaneko, S.; Arima, H. Anti-allergic effects of novel sulfated polysaccharide sacran on mouse model of 2,4-Dinitro-1-fluorobenzene-induced atopic dermatitis. Int. J. Biol. Macromol. 2018, 108, 112–118. [Google Scholar] [CrossRef]
- Puluhulawa, L.E.; Joni, I.M.; Mohammed, A.F.A.; Arima, H.; Wathoni, N. The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications. Molecules 2021, 26, 3362. [Google Scholar] [CrossRef]
- Matsuda, S.; Sugawa, H.; Shirakawa, J.; Ohno, R.; Kinoshita, S.; Ichimaru, K.; Arakawa, S.; Nagai, M.; Kabata, K.; Nagai, R. Aphanothece sacrum (Sur.) Okada Prevents Cataractogenesis in Type 1 Diabetic Mice. J. Nutr. Sci. Vitaminol. 2017, 63, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Gombos, Z.; Murata, N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature 1990, 347, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yi, W.; Song, J.K.; Wang, P.G. Current understanding on biosynthesis of microbial polysaccharides. Curr. Top. Med. Chem. 2008, 8, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P. (Ed.) Pseudomonas: Genomics and Molecular Biology, 1st ed.; Caister Academic Press: Brussel, Belgium, 2008. [Google Scholar] [CrossRef]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial Role of Extracellular Polysaccharides in Desiccation and Freezing Tolerance in the Terrestrial Cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.R.; Keenan, T.W.; Helm, R.F.; Potts, M.; Crowe, L.M.; Crowe, J.H. Extracellular polysaccharide of Nostoc commune (Cyanobacteria) inhibits fusion of membrane vesicles during desiccation. J. Appl. Phycol. 1997, 9, 237–248. [Google Scholar] [CrossRef]
- Okajima, M.K.; Sornkamnerd, S.; Kaneko, T. Development of Functional Bionanocomposites using Cyanobacterial Polysaccharides. Chem. Rec. 2018, 18, 1167–1177. [Google Scholar] [CrossRef]
- Julian, J.D.; Zabotina, O.A. Xyloglucan Biosynthesis: From Genes to Proteins and Their Functions. Front. Plant Sci. 2022, 13, 920494. [Google Scholar] [CrossRef] [PubMed]
- Dhara, S.; Chenchula, S.R.; Chakraborty, K.; Valluru, L.; Surabhi, G. Sulfated rhamnoglucan heteropolysaccharide of Spirulina platensis attenuates methimazole-induced hypothyroidism in rats. Algal Res. 2024, 78, 103409. [Google Scholar] [CrossRef]
- Yano, T.; Higaki, Y.; Tao, D.; Murakami, D.; Kobayashi, M.; Ohta, N.; Koike, J.; Horigome, M.; Masunaga, H.; Ogawa, H.; et al. Orientation of poly(vinyl alcohol) nanofiber and crystallites in non-woven electrospun nanofiber mats under uniaxial stretching. Polymer 2012, 53, 4702–4708. [Google Scholar] [CrossRef]






| Water | MeOH | EtOH | Acetone | Ethylene Glycol | 1,2-Propanediol | Glycerol | DMSO |
|---|---|---|---|---|---|---|---|
| + | − | − | − | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitani, C.; Okajima, M.; Ohashira, T.; Ali, M.A.; Taniike, T.; Kaneko, T. Structural Analyses of Polysaccharides Extracted from Cyanobacterial Extracellular Gels and Oriented Liquid Crystalline Microfiber Processing by Poly(vinyl alcohol)-Assisted Electrospinning. Gels 2024, 10, 321. https://doi.org/10.3390/gels10050321
Mitani C, Okajima M, Ohashira T, Ali MA, Taniike T, Kaneko T. Structural Analyses of Polysaccharides Extracted from Cyanobacterial Extracellular Gels and Oriented Liquid Crystalline Microfiber Processing by Poly(vinyl alcohol)-Assisted Electrospinning. Gels. 2024; 10(5):321. https://doi.org/10.3390/gels10050321
Chicago/Turabian StyleMitani, Chizu, Maiko Okajima, Tomomi Ohashira, Mohammad Asif Ali, Toshiaki Taniike, and Tatsuo Kaneko. 2024. "Structural Analyses of Polysaccharides Extracted from Cyanobacterial Extracellular Gels and Oriented Liquid Crystalline Microfiber Processing by Poly(vinyl alcohol)-Assisted Electrospinning" Gels 10, no. 5: 321. https://doi.org/10.3390/gels10050321






