Transcriptome Analysis on the Underlying Physiological Mechanism of Calcium and Magnesium Resolving “Sugar Receding” in ‘Feizixiao’ Litchi Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Materials
2.2. Extraction and Determination of Glucose, Fructose, and Sucrose
2.3. RNA Isolation and Sequencing
2.4. Sequence Assembly
2.5. Gene Annotation and Differentially Expressed Genes (DEGs) Screening
2.6. DEGs Enrichment Analysis
2.7. Primer Design and Real-Time PCR Verification
2.8. Data Analysis
3. Results and Analysis
3.1. Changes in Fructose, Glucose, Sucrose, and Soluble Sugar Contents
3.2. Quality Assessment and Assembly of Transcriptome Sequencing Result
3.3. Unigene Annotation Results
3.4. Quantification and KEGG and GO Enrichment Analyses of DEGs
3.5. Screening and Expression Pattern Analysis of Sugar Metabolism-Related Genes
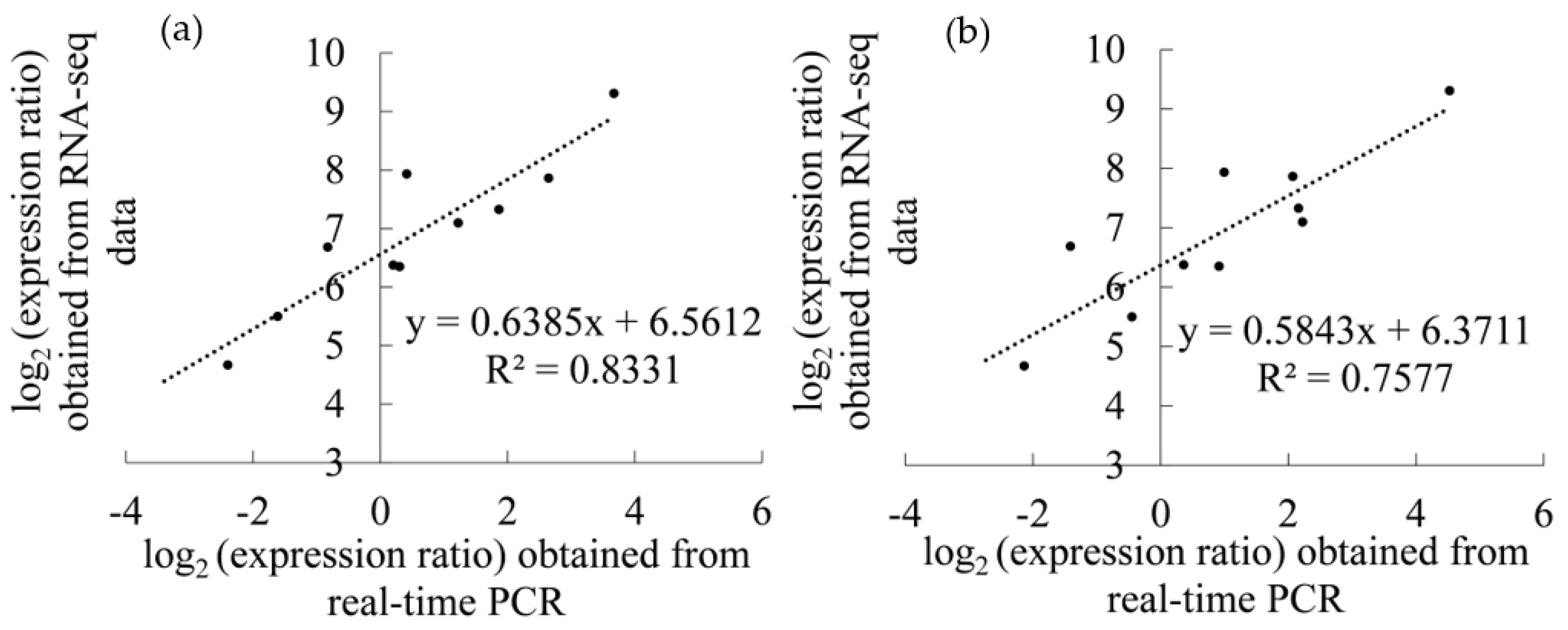
3.6. Real-Time PCR Validation
4. Discussion
4.1. The Ca and Mg Foliar Fertilizer Increased the Sugar Content in ‘Feizixiao’ Litchi Pulp
4.2. Ca and Mg Foliar Fertilizer Regulates the Sugar Content in ‘Feizixiao’ Litchi Pulp by Altering the Expression of Sugar Metabolism-Related Enzyme Genes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menzel, C. The physiology of growth and cropping in lychee. Acta Hortic. 2001, 558, 175–184. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, M.L.; Li, S.J.; Gao, D.; Zhou, K.B. Applications of Mg affect pericarp colour in the Feizixiao lychee. J. Hortic. Sci. Biotechnol. 2017, 92, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Huang, H.B.; Huang, X.M.; Hu, Z.Q. Sugar and acid compositions in the arils of Litchi chinensis Sonn.: Cultivar differences and evidence for the absence of succinic acid. J. Hortic. Sci. Biotechnol. 2006, 81, 57–62. [Google Scholar] [CrossRef]
- Braun, D.M.; Wang, L.; Ruan, Y.L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Das, N. An isoform of sucrose synthase involved in sink strength of potato (Solanum tuberosum L.): Molecular cloning, sequence analyses, 3-D structure, crucial motifs and expression. S. Afr. J. Bot. 2022, 149, 446–457. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Kelly, G.; Stein, O.; David-Schwartz, R. Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. J. Exp. Bot. 2014, 65, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera-Alvarado, G.P.; Sánchez-Nieto, S. Plant hexokinases are multifaceted proteins. Plant Cell Physiol. 2017, 58, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Granot, D. Putting plant hexokinases in their proper place. Phytochemistry 2008, 69, 2649–2654. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, A.; Laxmi, A. Glucose control of root growth direction in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 2981–2993. [Google Scholar] [CrossRef]
- Zhao, B.; Qi, K.; Yi, X.; Chen, G.; Liu, X.; Qi, X.; Zhang, S. Identification of hexokinase family members in pear (Pyrus × bretschneideri) and functional exploration of PbHXK1 in modulating sugar content and plant growth. Gene 2019, 711, 143932. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, A.; Jawandha, S.K.; Gill, P.; Singh, H. Influence of pre-harvest sprays of Ca nitrate on storability and quality attributes of plum fruits. J. Food Sci. Technol. 2019, 56, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Recasens, I.; Benavides, A.; Puy, J.; Casero, T. Pre-harvest Ca treatments in relation to the respiration rate and ethylene production of ‘Golden Smoothee’ apples. J. Sci. Food Agric. 2004, 84, 765–771. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Long, L.E. The effect of postharvest Ca application in hydro-cooling water on tissue Ca content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem. 2014, 160, 22–30. [Google Scholar] [CrossRef]
- Balic, I.; Ejsmentewicz, T.; Sanhueza, D.; Silva, C.; Peredo, T.; Olmedo, P.; Barros, M.; Verdonk, J.C.; Paredes, R.; Meneses, C.; et al. Biochemical and physiological study of the firmness of table grape berries. Postharvest Biol. Physiol. 2014, 93, 15–23. [Google Scholar] [CrossRef]
- Khaliq, G.; Mohamed, T.M.M.; Ali, A.; Ding, P.; Ghazali, H.M. Effect of gum arabic coating combined with Ca chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Wang, Y.; Long, L.E. Physiological and biochemical changes relating to postharvest splitting of sweet cherries affected by Ca application in hydrocooling water. Food Chem. 2015, 181, 241–247. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and Mg deficiency in bean plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.; Faucher, M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Mg deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef]
- Farhat, N.; Khouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of Mg deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of Mg in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Z.; Lin, X.K.; Du, J.J.; Peng, J.J.; Zhou, K.B. Transcriptomic analysis reveals key genes regulating organic acid synthesis and accumulation in the pulp of Litchi chinensis Sonn. cv. Feizixiao. Sci. Hortic. 2022, 303, 111220. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, T.D.; Wang, H.C.; Huang, X.M.; Qin, Y.H.; Hu, G.B. Patterns of enzyme activities and gene expressions in sucrose metabolism in relation to sugar accumulation and composition in the aril of Litchi chinensis Sonn. J. Plant Physiol. 2013, 170, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.; Haas, B.; Yassour, M.; Levin, J.; Thompson, D.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [Green Version]
- Polashock, J.; Arora, R.; Peng, Y.; Naik, D.; Rowland, L. Functional identification of a C-repeat binding factor transcriptional activator from blueberry associated with cold acclimation and freezing tolerance. J. Am. Soc. Hortic. Sci. 2010, 135, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Okubo, H.; Saitou, K. Increase in the expression of an alpha-amylase gene and sugar accumulation induced during cold period reflects shoot elongation in hyacinth bulbs. J. Am. Soc. Hortic. Sci. 2006, 131, 185–191. [Google Scholar] [CrossRef]
- Boyle, E.I.; Weng, S.; Gollub, J.; Jin, H.; Botstein, D.; Cherry, J.M.; Sherlock, G. GO: TermFinder—Open source software for accessing gene ontology information and finding significantly enriched gene ontology terms associated with a list of genes. Bioinformatics 2004, 20, 3710–3715. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Stoeckert, C.; Roos, D. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef]
- Natale, D.; Shankavaram, U.; Galperin, M.; Wolf, Y.; Aravind, L.; Koonin, E. Towards understanding the first genome sequence of a crenarchaeon by genome annotation using clusters of orthologous groups of proteins (COGs). Genome Biol. 2000, 1, RESEARCH0009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donovan, C.; Martin, M.; Gattiker, A.; Gasteiger, E.; Bairoch, A.; Apweiler, R. High-quality protein knowledge resource: SWISS-PROT and TrEMBL. Brief. Bioinform. 2002, 3, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Birney, E.; Cerruti, L.; Durbin, R.; Etwiller, L.; Eddy, S.; Jones, S.; Howe, K.; Marshall, M.; Sonnhammer, E. The Pfam protein families database. Nucleic Acids Res. 2002, 30, 276–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, D.; Clements, J.; Eddy, S. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.; Dillies, M. SARTools: A DESeq2- and edgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, W.W.; Situ, J.J.; Chen, Y.B.; Yang, X.Y.; Kong, G.H.; Liu, Y.Y.; Tinashe, R.J.; Xi, P.G.; Wang, Y.C.; et al. A Puf RNA-binding protein encoding gene PlM90 regulates the sexual and asexual life stages of the litchi downy blight pathogen Peronophythora litchii. Fungal Genet. Biol. 2017, 98, 39–45. [Google Scholar] [CrossRef]
- Liaquat, M.; Ahmad, S.; Khan, A.S.; Ahmed, R. Reduction in fruit rot and enhancement in fruit quality of kinnow mandarin by Ca chloride application. Pak. J. Agric. Sci. 2019, 56, 367–376. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Mg fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.W.; Yang, W.H.; Muneer, M.A.; Ji, Z.J.; Tong, L.; Zhang, X.; Li, X.X.; Wang, W.Q.; Zhang, F.S.; Wu, L.Q. Integrated use of lime with Mg fertilizer significantly improves the pomelo yield, quality, economic returns and soil physicochemical properties under acidic soil of southern China. Sci. Hortic. 2021, 290, 110502. [Google Scholar] [CrossRef]
- Xu, D.H.; Carswell, A.; Zhu, Q.C.; Zhang, F.S.; Vries, W.D. Modelling long-term impacts of fertilization and liming on soil acidification at Rothamsted experimental station. Sci. Total Environ. 2020, 713, 136249. [Google Scholar] [CrossRef]
- D’Aoust, M.A.; Yelle, S.; Nguyen-Quoc, B. Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and sucrose unloading capacity of young fruit. Plant Cell 1999, 11, 2407–2418. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Li, L.M.; Yang, P.P.; Gong, C.L. The role of hexokinases from grape berries (Vitis vinifera L.) in regulating the expression of cell wall invertase and sucrose synthase genes. Plant Cell Rep. 2014, 33, 337–347. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Sheen, J. Master regulators in plant glucose signaling networks. J. Plant Biol. 2014, 57, 67–79. [Google Scholar] [CrossRef]





| Primer Names | Left Primer Sequences (5′ to 3′) | Right Primer Sequences (5′ to 3′) | Primer Efficiency |
|---|---|---|---|
| Cluster-6206.78756 | CTGCCTCCATTTGTGGCTAT | CAAAATCAAGCTCGAGCACA | 1.097 |
| Cluster-6206.79539 | TGGGTTTTGAGAAAGGTTGG | CATCGGTACTCTCCCAAGGA | 1.090 |
| Cluster-6206.80820 | GACTTGTGGCCTTCCAACAT | ATTTTATGCCAGTGGCTTGG | 1.078 |
| Cluster-6206.70575 | AAGCTTTGCTCAAGGTGGAA | GTCATTGGAGAAGGGACGA | 1.028 |
| Cluster-6206.77750 | TCCTCCTTCATTGGGTCAAG | CCGATTGTGATCCTCCACTT | 1.069 |
| Cluster-6206.71042 | GGGAGAAGTCGTACGCAGAG | TTTTCAGTTTCGGTGGAACC | 1.083 |
| Cluster-6206.88230 | GTCGCCCAAAAGATTGTTGT | GCAAAGACCACCACACGTTA | 1.074 |
| Cluster-6206.71164 | GGGGAGCTTCTCAGCTTTTT | CCAGTGCCCTTCATACCAGT | 1.086 |
| Cluster-6206.79974 | TCTATCTGGGCATGGGAGTC | AAGCTTCAAAGGACCCACCT | 1.061 |
| Cluster-6206.77879 | AATCTTGCCCTCAACATTGC | GAGCACCAGCCTTAACAAGC | 0.990 |
| actin | AGTTTGGTTGATGTGGGAGAC | TGGCTGAACCCGAGATGAT | 1.085 |
| Sample | Raw Reads | Clean Reads | Clean Base (G) | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|
| A1-1 | 46,959,760 | 45,551,644 | 6.83 | 0.02 | 98.30 | 94.88 | 47.25 |
| A1-2 | 44,303,098 | 43,024,646 | 6.45 | 0.02 | 98.34 | 95.00 | 45.96 |
| A1-3 | 46,957,052 | 45,774,790 | 6.87 | 0.02 | 98.29 | 94.86 | 45.91 |
| A2-1 | 42,158,766 | 40,912,018 | 6.14 | 0.02 | 98.40 | 95.04 | 45.12 |
| A2-2 | 42,572,238 | 41,238,516 | 6.19 | 0.02 | 98.50 | 95.33 | 45.35 |
| A2-3 | 45,151,584 | 43,462,948 | 6.52 | 0.02 | 98.35 | 94.98 | 45.43 |
| B2-1 | 42,733,518 | 41,547,074 | 6.23 | 0.02 | 98.34 | 94.98 | 45.19 |
| B2-2 | 52,085,004 | 50,219,550 | 7.53 | 0.02 | 98.35 | 94.99 | 45.45 |
| B2-3 | 50,267,754 | 48,917,760 | 7.34 | 0.02 | 98.21 | 94.59 | 45.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Du, J.; Ma, W.; Chen, T.; Shui, X.; Liao, H.; Lin, X.; Zhou, K. Transcriptome Analysis on the Underlying Physiological Mechanism of Calcium and Magnesium Resolving “Sugar Receding” in ‘Feizixiao’ Litchi Pulp. Horticulturae 2022, 8, 1197. https://doi.org/10.3390/horticulturae8121197
Peng J, Du J, Ma W, Chen T, Shui X, Liao H, Lin X, Zhou K. Transcriptome Analysis on the Underlying Physiological Mechanism of Calcium and Magnesium Resolving “Sugar Receding” in ‘Feizixiao’ Litchi Pulp. Horticulturae. 2022; 8(12):1197. https://doi.org/10.3390/horticulturae8121197
Chicago/Turabian StylePeng, Junjie, Jingjia Du, Wuqiang Ma, Tiantian Chen, Xian Shui, Haizhi Liao, Xiaokai Lin, and Kaibing Zhou. 2022. "Transcriptome Analysis on the Underlying Physiological Mechanism of Calcium and Magnesium Resolving “Sugar Receding” in ‘Feizixiao’ Litchi Pulp" Horticulturae 8, no. 12: 1197. https://doi.org/10.3390/horticulturae8121197





