Exploring Spontaneous Combustion Characteristics and Structural Disparities of Coal Induced by Igneous Rock Erosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Information
2.2. Analysis Methods
3. Results and Discussion
3.1. Gas Change Rate and Spontaneous Combustion Parameters
3.1.1. Gas Change Rate
3.1.2. Analysis of Spontaneous Combustion Limit Parameters
3.2. Kinetic Parameters of Coal Oxidation
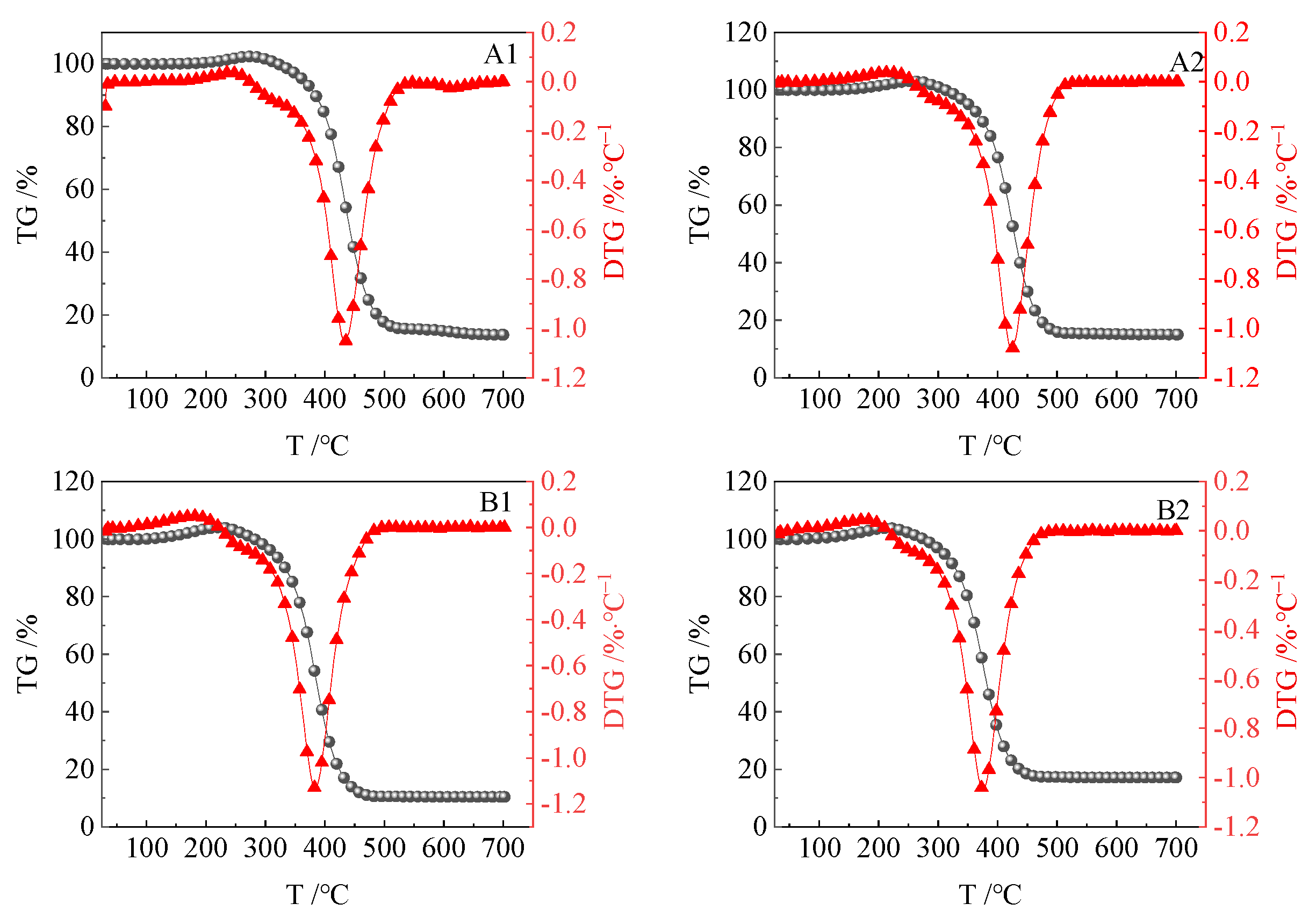
3.2.1. Characteristic Temperature Analysis
3.2.2. Activation Energy Analysis
3.3. FT-IR Analysis of Coal Samples
3.3.1. Functional Group Content Analysis
3.3.2. Chemical Structure Analysis of Coal
3.4. Specific Surface Area and Pore Distribution of Coal
3.5. Difference Analysis of Spontaneous Combustion of Igneous Eroded Coal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Wu, S.; Lv, Q.; Liu, X.; Chen, W.; Che, D. Study on correlations of coal chemical properties based on database of real-time data. Appl. Energy 2017, 204, 1115–1123. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, F.-B. A comprehensive hazard evaluation system for spontaneous combustion of coal in underground mining. Int. J. Coal Geol. 2010, 82, 27–36. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Cheng, Y.; Zheng, S.; Zhang, S.; Wang, R. Numerical simulation of magma intrusion on the thermal evolution of low-rank coal. Environ. Earth Sci. 2021, 80, 562. [Google Scholar] [CrossRef]
- Dutcher, R.R.; Campbell, D.-L.; Thornton, C.P. Coal metamorphism and igneous intrusions in Colorado. Adv. Chem. 1966, 55, 708–723. [Google Scholar] [CrossRef]
- Finkelman, R.-B.; Bostick, N.-H.; Dulong, F.-T.; Senftle, F.E.; Thorpe, A.N. Influence of an igneous intrusion on the inorganic geochemistry of a bituminous coal from Pitkin County, Colorado. Int. J. Coal Geol. 1998, 36, 223–241. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, L.-B.; Cheng, Y.-P.; Yin, G.-Z.; Cai, C.-C.; Xu, C.; Jin, K. Thermal effects of magmatic sills on coal seam metamorphism and gas occurrence. Bull. Volcanol. 2014, 76, 1411–1422. [Google Scholar] [CrossRef]
- Aarnes, I.; Svensen, H.; Polteau, S.; Planke, S. Contact metamorphic devolatilization of shales in the Karoo Basin, South Africa, and the effects of multiple sill intrusions. Chem. Geol. 2011, 281, 181–194. [Google Scholar]
- Gao, Y.; Qin, B.; Shi, Q.; Liang, H.; Chen, K. Effect of Igneous Intrusions on Low-temperature Oxidation Characteristics of Coal in Daxing Mine, China. Combust. Sci. Technol. 2021, 193, 577–593. [Google Scholar]
- Goodarzi, F.; Gentzis, T.; Grasby, S.-E.; Dewing, K. Influence of igneous intrusions on thermal maturity and optical texture: Comparison between a bituminous marl and a coal seam of the same maturity. Int. J. Coal Geol. 2018, 198, 183–197. [Google Scholar]
- Rahman, M.W.; Rimmer, S.M. Effects of rapid thermal alteration on coal: Geochemical and petrographic signatures in the Springfield (No. 5) Coal, Illinois Basin. Int. J. Coal Geol. 2014, 131, 214–226. [Google Scholar] [CrossRef]
- Qin, Y.; Jin, K.; Tian, F.; Su, W.; Ren, S. Effects of ultrathin igneous sill intrusion on the petrology, pore structure and ad/desorption properties of high volatile bituminous coal: Implications for the coal and gas outburst prevention. Fuel 2022, 316, 123340. [Google Scholar] [CrossRef]
- He, J.; Wang, B.; Lu, Z. Experimental study on the effect of magma intrusion and temperature on the pore structure of coal. Energy 2023, 284, 128688. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Cheng, Y.; Jin, K.; Zhao, W.; Guo, H. Influence of thermal metamorphism on CBM reservoir characteristics of low-rank bituminous coal. J. Nat. Gas Sci. Eng. 2016, 36, 916–930. [Google Scholar] [CrossRef]
- Wang, D.; Song, Y.; Xu, H.; Ma, X.; Zhao, M. Numerical modeling of thermal evolution in the contact aureole of a 0.9 m thick dolerite dike in the Jurassic siltstone section from Isle of Skye, Scotland. J. Appl. Geophys. 2013, 89, 134–140. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Effects of igneous intrusions on coal petrology, pore-fracture and coalbed methane characteristics in Hongyang, Handan and Huaibei coalfields, North China. Int. J. Coal Geol. 2012, 96–97, 72–81. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, L.-B.; Cheng, Y.-P.; Yin, G.-Z.; Xu, C.; Jin, K.; Yang, Q.-L. Characteristics and evolutions of gas dynamic disaster under igneous intrusions and its control technologies. J. Nat. Gas Sci. Eng. 2014, 18, 164–174. [Google Scholar] [CrossRef]
- Li, J.; Zhou, F.; Liu, Y. Effect of magmatic intrusion on coal pore characteristics and fractal research. J. Min. Sci. 2015, 51, 743–754. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Chen, S.; Wang, H. Response of coal reservoir porosity to magma intrusion in the Shandong Qiwu Mine, China. Min. Sci. Technol. 2011, 21, 185–190. [Google Scholar] [CrossRef]
- Cao, D.; Zhan, W.-F.; Li, X.-M. Effect of intrusion of the Shangcheng granite body on structural framework of the Yangshan coal-bearing series, the Beihuaiyang area, China. J. China Univ. Min. Technol. 2007, 36, 320–324. [Google Scholar]
- Shi, Q.; Qin, B.; Liang, H.; Gao, Y.; Bi, Q.; Qu, B. Effects of igneous intrusions on the structure and spontaneous combustion propensity of coal: A case study of bituminous coal in Daxing Mine, China. Fuel 2018, 216, 181–189. [Google Scholar] [CrossRef]
- Singh, R.-N.; Shonhardt, J.-A.; Terezopoulos, N. A new dimension to studies of spontaneous combustion of coal. Miner. Resour. Eng. 2002, 11, 147–163. [Google Scholar] [CrossRef]
- Ma, L.; Zou, L.; Ren, L.-F.; Chung, Y.-H.; Zhang, P.-Y.; Shu, C.-M. Prediction indices and limiting parameters of coal spontaneous combustion in the Huainan mining area in China. Fuel 2020, 264, 116883. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, J.; Chen, L.; Wang, T.; Song, J.; Zhang, Y.; Shu, C.-M.; Zeng, Q. Correlation analysis of the functional groups and exothermic characteristics of bituminous coal molecules during high-temperature oxidation. Energy 2019, 181, 136–147. [Google Scholar]
- Deng, C.-B.; Shan, Y.-F.; Hong, L.; Lu, W.-D. New classifying method of the spontaneous combustion tendency. J. China Coal Soc. 2008, 33, 47–50. [Google Scholar]
- Pan, R.-K.; Li, C.; Yu, M.-G.; Xiao, Z.-J.; Fu, D. Evolution patterns of coal micro-structure in environments with different temperatures and oxygen conditions. Fuel 2019, 261, 116425. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Zhao, J.; Deng, J.; Yang, H. Evaluation of the spontaneous combustion characteristics of coal of different metamorphic degrees based on a temperature-programmed oil bath experimental system. J. Loss Prev. Process -Dustries 2019, 60, 17–27. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Zhang, G.; Zhao, Y.; Zhu, P.; Ma, X.; Liu, X. Effect of multi-component gases competitive adsorption on coal spontaneous combustion characteristics under goaf conditions. Fuel Process. Technol. 2020, 208, 106510. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Xue, S.; Wang, J.; Zhang, Y. Experimental Study on the Molecular Hydrogen Release Mechanism during Low-Temperature Oxidation of Coal. Energy Fuels 2017, 31, 5498–5506. [Google Scholar] [CrossRef]
- Zhong, X.; Kan, L.; Xin, H.; Qin, B.; Dou, G. Thermal effects and active group differentiation of low-rank coal during low-temperature oxidation under vacuum drying after water immersion. Fuel 2019, 236, 1204–1212. [Google Scholar] [CrossRef]
- Li, K.; Khanna, R.; Zhang, J.; Barati, M.; Liu, Z.; Xu, T.; Yang, T.; Sahajwalla, V. Comprehensive Investigation of Various Structural Features of Bituminous Coals Using Advanced Analytical Techniques. Energy Fuels 2015, 29, 7178–7189. [Google Scholar] [CrossRef]
- GB/T 474-2008; Method for preparation of coal sample. National Coal Standardization Technical Committee: Beijing, China, 2008.
- Su, H.; Zhou, F.; Li, J.; Qi, H. Effects of oxygen supply on low-temperature oxidation of coal: A case study of Jurassic coal in Yima, China. Fuel 2017, 202, 446–454. [Google Scholar] [CrossRef]
- Wang, G.; Yan, G.; Zhang, X.; Du, W.; Huang, Q.; Sun, L.; Zhang, X. Research and development of foamed gel for controlling the spontaneous combustion of coal in coal mine. J. Loss Prev. Process. Ind. 2016, 44, 474–486. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Ryu, C.; Park, H.Y.; Lim, H. Low-temperature reactivity of coals for evaluation of spontaneous combustion propensity. Korean J. Chem. Eng. 2015, 32, 1297–1304. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Xue, S.; Wu, J.; Tang, Y.; Chang, L. Assessment of spontaneous combustion status of coal based on relationships between oxygen consumption and gaseous product emissions. Fuel Process. Technol. 2018, 179, 60–71. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Yang, Z.; Liu, Y.; Yu, J. Exothermic characteristics of coal during low-temperature oxidation based on grey correlation method. Energy Rep. 2022, 8, 6744–6752. [Google Scholar] [CrossRef]
- Xu, J.; Ge, L.; He, D. Study on the process of low temperature spontaneous combustion of coal. Coal Eng. 1989, 5, 7–13. [Google Scholar]
- Yang, Y.; Li, Z.; Si, L.; Hou, S.; Li, Z.; Li, J. Study on test method of heat release intensity and thermophysical parameters of loose coal. Fuel 2018, 229, 34–43. [Google Scholar] [CrossRef]
- Wang, C.-P.; Zhao, X.-Y.; Bai, Z.-J.; Deng, J.; Shu, C.-M.; Zhang, M. Comprehensive index evaluation of the spontaneous combustion capability of different ranks of coal. Fuel 2021, 291, 120087. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, Y.; Li, Q.; Zhang, J.; Yang, C. Study on the characteristics of coal spontaneous combustion during the development and decaying processes. Process. Saf. Environ. Prot. 2020, 138, 9–17. [Google Scholar] [CrossRef]
- Yan, H.; Nie, B.; Kong, F.; Liu, Y.; Liu, P.; Wang, Y.; Chen, Z.; Yin, F.; Gong, J.; Lin, S.; et al. Experimental investigation of coal particle size on the kinetic properties of coal oxidation and spontaneous combustion limit parameters. Energy 2023, 270, 126890. [Google Scholar] [CrossRef]
- Xin, Y.I.; Bai, Z.J.; Xiao, Y.; Wang, C.P.; Deng, J.; Chen, L.G. Impact of imidazole ionic liquid on the maximal limit parameters of the coal spontaneous combustion. J. Saf. Environ. 2018, 18, 1805–1810. [Google Scholar] [CrossRef]
- Tan, B.; Hu, R.; Li, K.; Bao, T.; Fan, W. Comparison analysis on spontaneous combustion limit parameters of bituminous coal with different metamorphic degree. Coal Sci. Technol. 2013, 41, 63–67. [Google Scholar] [CrossRef]
- Zhou, F.; Ren, W.; Wang, D.; Song, T.; Li, X.; Zhang, Y. Application of three-phase foam to fight an extraordinarily serious coal mine fire. Int. J. Coal Geol. 2006, 67, 95–100. [Google Scholar] [CrossRef]
- Li, Q.-W.; Xiao, Y.; Wang, C.-P.; Deng, J.; Shu, C.-M. Thermokinetic characteristics of coal spontaneous combustion based on thermogravimetric analysis. Fuel 2019, 250, 235–244. [Google Scholar] [CrossRef]
- Xi, Z.; Shan, Z.; Li, M.; Wang, X. Analysis of Coal Spontaneous Combustion by Thermodynamic Methods. Combust. Sci. Technol. 2020, 193, 2305–2330. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Zhang, H.; Xin, H. Thermodynamic characteristics of coal reaction under low oxygen concentration conditions. J. Energy Inst. 2017, 90, 544–555. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Xie, H.; Qin, Q. Pyrolysis of coal by solid heat carrier-experimental study and kinetic modeling. Energy 2017, 135, 317–326. [Google Scholar] [CrossRef]
- Wang, R.; Liu, G. Variations of concentration and composition of polycyclic aromatic hydrocarbons in coals in response to dike intrusion in the Huainan coalfield in eastern China. Org. Geochem. 2015, 83–84, 202–214. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, J.; Lu, B.; Huang, G.; Yu, C.; Liang, H. Experimental study on effect of mudstone on spontaneous combustion of coal. Energy 2023, 285, 128784. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Y.; Wang, L.; Zhou, H. Experiments on the effects of igneous sills on the physical properties of coal and gas occurrence. J. Nat. Gas Sci. Eng. 2014, 19, 98–104. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.H.; Guo, Y.; Su, Y. FTIR and 13C NMR Investigation of Coal Component of Late Permian Coals from Southern China. Energy Fuels 2011, 25, 5672–5677. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Zhang, Y.-L.; Song, J.-J.; Zhang, T.-H.; Ming, H.-Q.; Lu, S.-P.; Deng, J.; Shu, C.-M. Microstructure of coal spontaneous combustion in low-oxygen atmospheres at characteristic temperatures. Fuel 2021, 309, 122132. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Bi, Q.; Qu, B. An experimental study on the effect of igneous intrusions on chemical structure and combustion characteristics of coal in Daxing Mine, China. Fuel 2018, 226, 307–315. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, Y.; Wang, Z.; Zhou, J.; Cen, K. Effects of microwave irradiation treatment on physicochemical characteristics of Chinese low-rank coals. Energy Convers. Manag. 2013, 71, 84–91. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, W.; Hu, Y.; Meng, Q. Study of micro-pores development in lean coal with temperature. Int. J. Rock Mech. Min. Sci. Géoméch. Abstr. 2012, 51, 91–96. [Google Scholar] [CrossRef]
- Jiang, J.-Y.; Cheng, Y.-P.; Wang, L.; Li, W.; Wang, L. Petrographic and geochemical effects of sill intrusions on coal and their implications for gas outbursts in the Wolonghu Mine, Huaibei Coalfield, China. Int. J. Coal Geol. 2011, 88, 55–66. [Google Scholar] [CrossRef]
- Dias, R.-F.; Lewan, M.-D.; Birdwell, J.-E.; Kotarba, M.J. Differentiation of pre-existing trapped methane from thermogenic methane in an igneous-intruded coal by hydrous pyrolysis. Org. Geochem. 2014, 67, 1–7. [Google Scholar] [CrossRef]
- Karsner, G.-G.; Perlmutter, D.-D. Reaction regimes in coal oxidation. AIChE J. 1981, 27, 920–927. [Google Scholar] [CrossRef]
- Kaji, R.; Hishinuma, Y.; Nakamura, Y. Low temperature oxidation of coals: Effects of pore structure and coal composition. Fuel 1985, 64, 297–302. [Google Scholar] [CrossRef]
- Tang, Y.; Xue, S. Laboratory Study on the Spontaneous Combustion Propensity of Lignite Undergone Heating Treatment at Low Temperature in Inert and Low-Oxygen Environments. Energy Fuels 2015, 29, 4683–4689. [Google Scholar] [CrossRef]
- Saghafi, A.; Faiz, M.; Roberts, D. CO2 storage and gas diffusivity properties of coals from Sydney Basin, Australia. Int. J. Coal Geol. 2007, 70, 240–254. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, Y. Effects of Igneous Intrusion on Microporosity and Gas Adsorption Capacity of Coals in the Haizi Mine, China. Sci. World J. 2014, 2014, 976512–976582. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cao, Y.-J.; Tien, J.C. Method for prevention and control of spontaneous combustion of coal seam and its application in mining field. Int. J. Min. Sci. Technol. 2017, 27, 839–846. [Google Scholar] [CrossRef]












| Coal Sample | Proximate Analysis/% | Elemental Analysis/% | H/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatiles | Fixed Carbon | C | H | O | N | S | ||
| A1 | 5.13 | 11.36 | 33.71 | 49.80 | 79.92 | 5.22 | 13.26 | 1.26 | 0.34 | 0.78 |
| A2 | 2.85 | 15.02 | 26.31 | 55.82 | 81.02 | 4.86 | 12.45 | 1.31 | 0.36 | 0.72 |
| B1 | 3.55 | 9.47 | 36.48 | 49.50 | 79.85 | 5.36 | 13.49 | 1.01 | 0.29 | 0.81 |
| B2 | 1.65 | 14.88 | 27.98 | 55.49 | 80.74 | 5.12 | 12.86 | 0.96 | 0.32 | 0.76 |
| Coal Sample | T0/°C | Ta/°C | Tb/°C | Tig/°C | Tmax/°C | Tc/°C |
|---|---|---|---|---|---|---|
| A1 | 31.19 | 113.09 | 273.23 | 387.96 | 432.86 | 549.45 |
| A2 | 31.59 | 68.04 | 260.96 | 380.63 | 422.92 | 544.63 |
| B1 | 31.44 | 74.02 | 222.63 | 335.41 | 380.07 | 514.97 |
| B2 | 31.27 | 63.16 | 212.23 | 328.36 | 375.55 | 503.06 |
| Coal Sample | E/KJ·mol−1 | R2 | Coal Sample | E/KJ·mol−1 | R2 |
|---|---|---|---|---|---|
| A1 | 66.1213 | 0.9767 | A2 | 57.6178 | 0.9790 |
| B1 | 52.0858 | 0.9740 | B2 | 35.9615 | 0.9831 |
| Coal Sample | Aromatic Hydrocarbon/% | Oxygen-Containing Functional Group/% | Aliphatic Hydrocarbon/% | Hydroxyl/% |
|---|---|---|---|---|
| A1 | 0.44 | 14.62 | 0.91 | 84.04 |
| A2 | 0.97 | 29.33 | 1.98 | 67.73 |
| B1 | 0.71 | 34.97 | 1.56 | 62.77 |
| B2 | 0.97 | 42.39 | 2.05 | 54.60 |
| Coal Sample | A900—700 | A(C=C) | A(C=O) | A(CH2) | A(CH3) | A3000—2800 |
|---|---|---|---|---|---|---|
| A1 | 61.5798 | 822.7195 | 333.6664 | 80.9893 | 14.2851 | 127.8715 |
| A2 | 145.94258 | 1167.4645 | 258.1762 | 182.4507 | 31.0385 | 299.6164 |
| B1 | 58.1469 | 470.3174 | 155.2843 | 77.6118 | 13.8259 | 128.1153 |
| B2 | 77.0079 | 574.4442 | 177.0478 | 96.6709 | 17.1441 | 163.3034 |
| Coal Sample | fa | DOC | CH2/CH3 | I |
|---|---|---|---|---|
| A1 | 0.7061 | 0.0748 | 5.6695 | 0.4816 |
| a2 | 0.7311 | 0.1250 | 5.8782 | 0.48714 |
| B1 | 0.6922 | 0.1236 | 5.6135 | 0.45390 |
| B2 | 0.7127 | 0.1341 | 5.6387 | 0.47156 |
| Coal Sample | BET | Pore Volume | ||
|---|---|---|---|---|
| Specific Surface Area/(m2·g−1) | Average Pore Diameter/nm | HK Micropore Volume/×10−3(cm3·g−1) | BJH Mesopore Volume/×10−3(cm3·g−1) | |
| A1 | 14.43 | 5.67 | 4.27 | 18.29 |
| A2 | 4.55 | 8.21 | 1.62 | 13.47 |
| B1 | 17.39 | 5.34 | 6.38 | 19.40 |
| B2 | 9.33 | 10.79 | 2.91 | 13.17 |
| Coal Sample | Specific Surface Area/(m2·g−1) | Pores Volume/(cm3·g−1) | ||
|---|---|---|---|---|
| Mesoporous | Macropores | Mesoporous | Macropores | |
| A1 | 22.1051 | 0.2780 | 0.0606 | 0.0109 |
| A2 | 17.3817 | 1.0347 | 0.0582 | 0.0325 |
| B1 | 17.9801 | 0.2377 | 0.0499 | 0.0072 |
| B2 | 14.1379 | 0.5602 | 0.0462 | 0.0212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, Z.; Chen, Z.; Gao, L.; Qi, Y.; Hu, H. Exploring Spontaneous Combustion Characteristics and Structural Disparities of Coal Induced by Igneous Rock Erosion. Fire 2024, 7, 159. https://doi.org/10.3390/fire7050159
Zhang M, Li Z, Chen Z, Gao L, Qi Y, Hu H. Exploring Spontaneous Combustion Characteristics and Structural Disparities of Coal Induced by Igneous Rock Erosion. Fire. 2024; 7(5):159. https://doi.org/10.3390/fire7050159
Chicago/Turabian StyleZhang, Mingqian, Zongxiang Li, Zhifeng Chen, Lun Gao, Yun Qi, and Haifeng Hu. 2024. "Exploring Spontaneous Combustion Characteristics and Structural Disparities of Coal Induced by Igneous Rock Erosion" Fire 7, no. 5: 159. https://doi.org/10.3390/fire7050159





