Evaluation of Almond Hull and Shell Amendments across Organic Matter Management of Orchard Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Incubation
2.2. Field Study
2.3. Sample Collection and Analysis
2.4. Statistical Analysis
3. Results
3.1. Total Soil Organic C and N
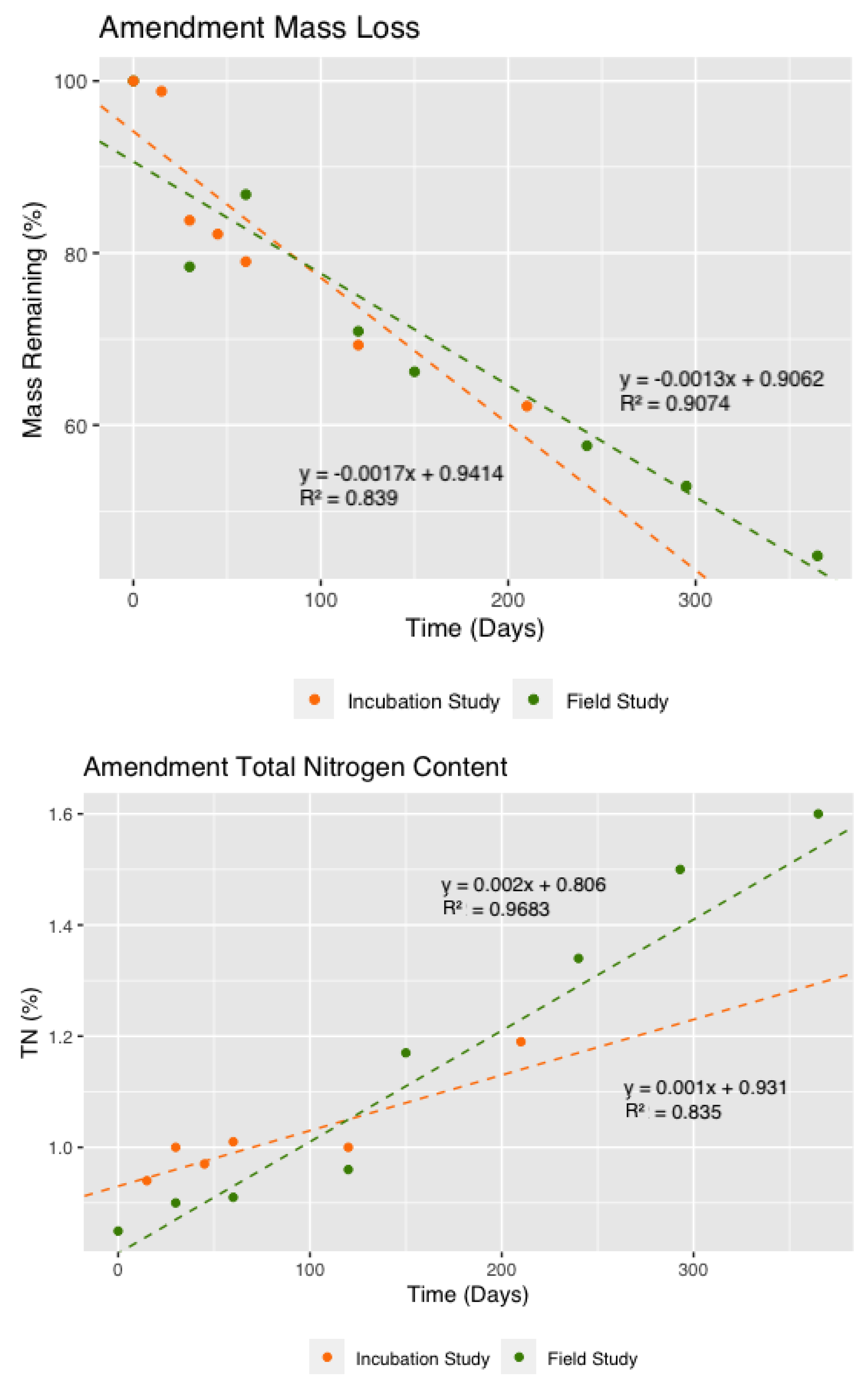
3.2. Decomposition and Nutrient Release from the Amendment Layer
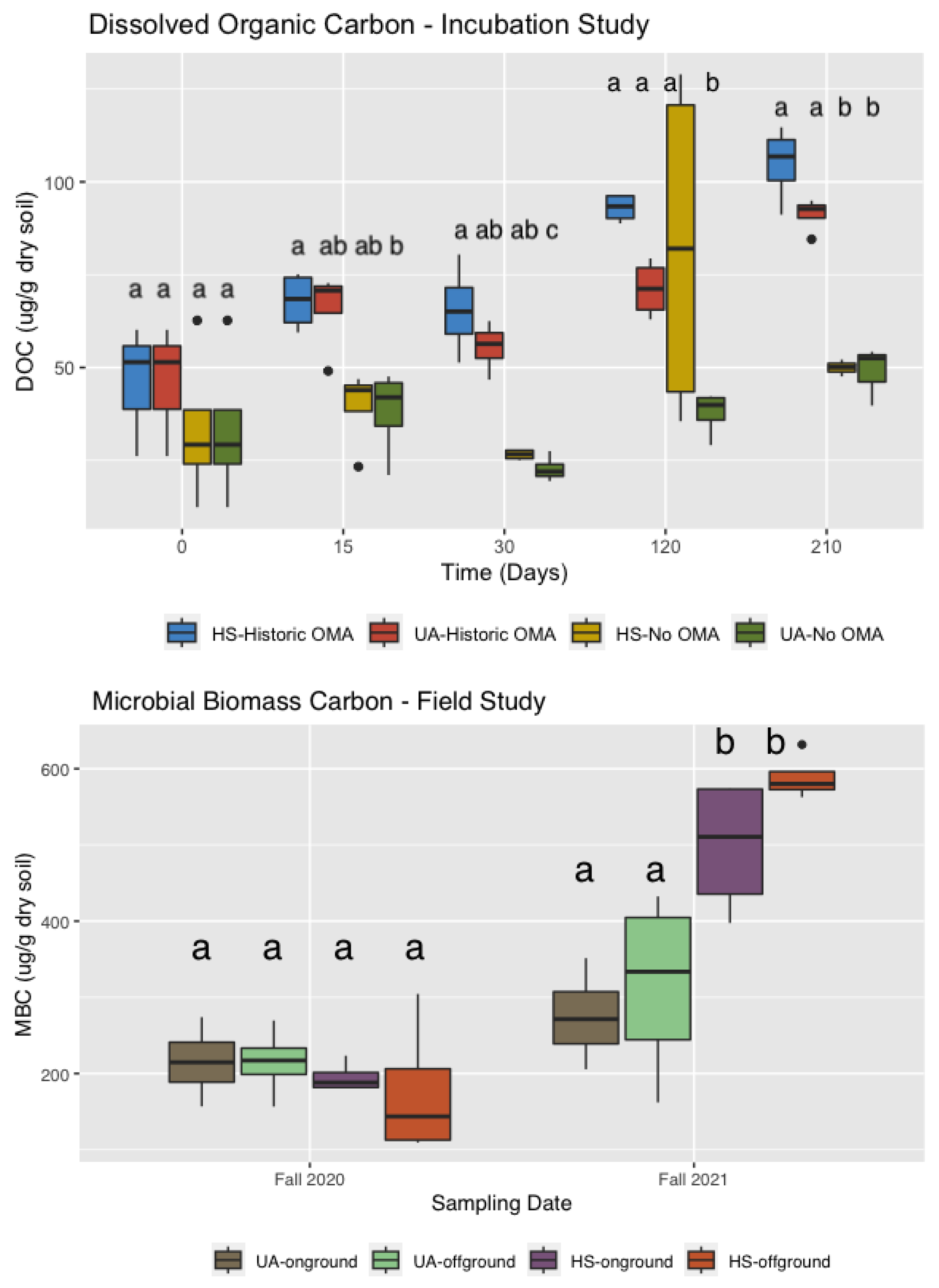
3.3. Dissolved Organic Carbon and Microbial Biomass
3.4. Cumulative Respiration
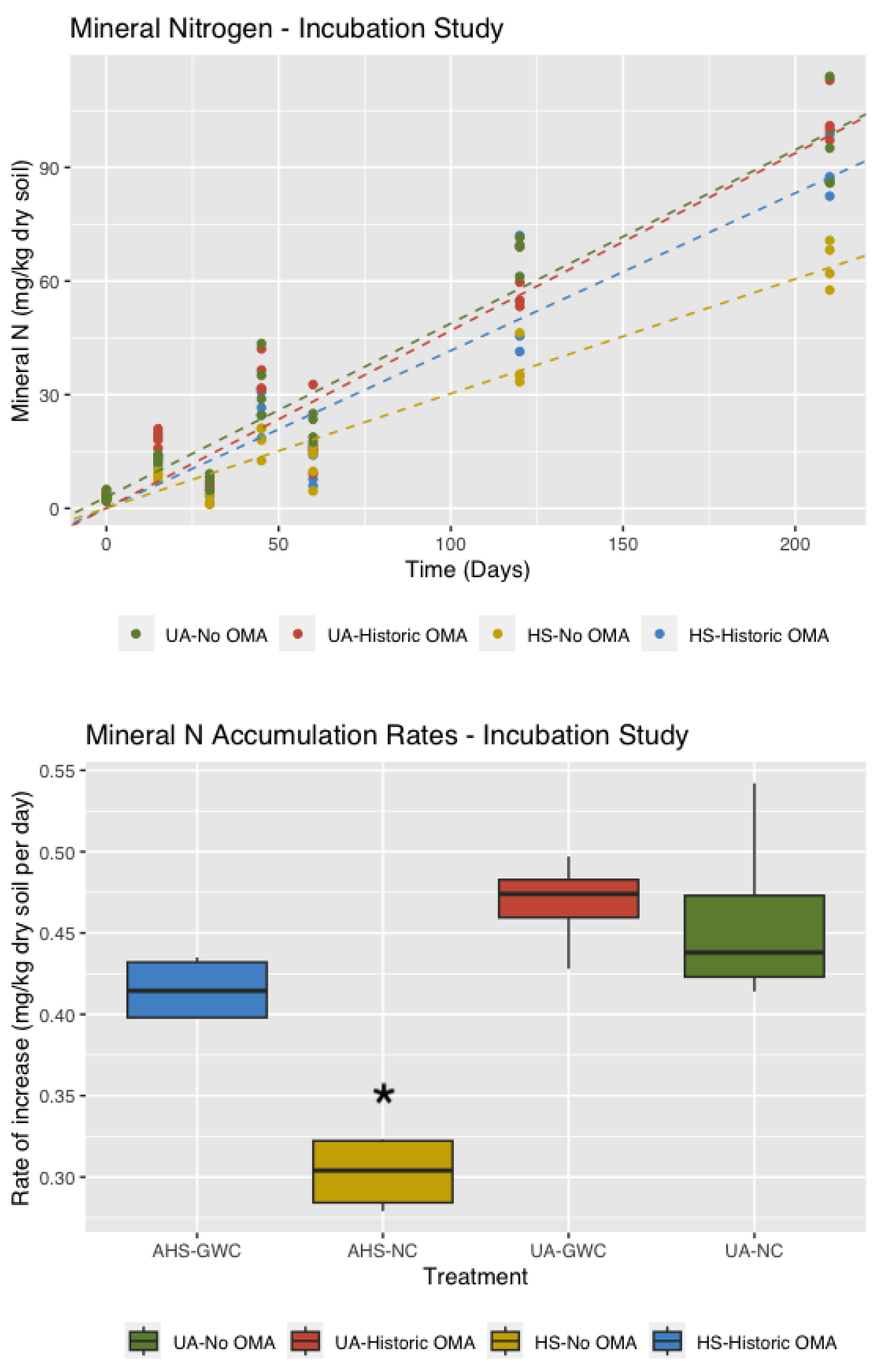
3.5. Mineral Nitrogen
4. Discussion
4.1. Impact of Soil History Highlights Long-Term Effects on C and N Cycling
4.2. Mineral N Accumulation Is Affected by Both History and Amendments
4.3. Microbial Biomass and Respiration Rapidly Respond to Amendments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Bayo, J.D.; Shea, E.A.; Parr, A.E.; Achmon, Y.; Stapleton, J.J.; VanderGheynst, J.S.; Hodson, A.K.; Simmons, C.W. Almond Processing Residues as a Source of Organic Acid Biopesticides during Biosolarization. Waste Manag. 2020, 101, 74–82. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. National Agricultural Statistics Service 2020 California Almond Acreage Report; Pacific Region: Sacramento, CA, USA, 2021.
- Muhammad, S.; Sanden, B.; Lampinen, B.; Saa, S.; Siddiqui, M.; Smart, D.; Olivos-Del Rio, A.; Shackel, K.; Dejong, T.; Brown, P. Seasonal Changes in Nutrient Content and Concentrations in a Mature Deciduous Tree Species: Studies in Almond (Prunus dulcis (Mill.) D. A. Webb). Europ. J. Agron. 2015, 65, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.M.; Rivers, D.J.; Gaudin, A.C.M.; Geisseler, D.; Brown, P.H.; Khalsa, S.D.S. In a Nutshell: Almond Hull and Shell Organic Matter Amendments Increase Soil and Tree Potassium Status. Plant Soil 2024, 495, 699–722. [Google Scholar] [CrossRef]
- Jahanzad, E.; Holtz, B.A.; Zuber, C.A.; Doll, D.; Brewer, K.M.; Hogan, S.; Gaudin, A.C.M. Orchard Recycling Improves Climate Change Adaptation and Mitigation Potential of Almond Production Systems. PLoS ONE 2020, 15, e0229588. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.D.S.; Hart, S.C.; Brown, P.H. Nutrient Dynamics from Surface-Applied Organic Matter Amendments on No-till Orchard Soil. Soil Use Manag. 2022, 38, 649–662. [Google Scholar] [CrossRef]
- Villa, Y.B.; Khalsa, S.D.S.; Ryals, R.; Duncan, R.A.; Brown, P.H.; Hart, S.C. Organic Matter Amendments Improve Soil Fertility in Almond Orchards of Contrasting Soil Texture. Nutr. Cycl. Agroecosyst. 2021, 120, 343–361. [Google Scholar] [CrossRef]
- Jafari, M.; Haghighi, J.A.P.; Zare, H. Mulching Impact on Plant Growth and Production of Rainfed Fig Orchards under Drought Conditions. J. Food Agric. AMP Environ. 2012, 10, 428–433. [Google Scholar] [CrossRef]
- Verdú, A.M.; Mas, M.T. Mulching as an Alternative Technique for Weed Management in Mandarin Orchard Tree Rows. Agron. Sustain. Dev. 2007, 27, 367–375. [Google Scholar] [CrossRef]
- López, R.; Burgos, P.; Hermoso, J.M.; Hormaza, J.I.; González-Fernández, J.J. Long Term Changes in Soil Properties and Enzyme Activities after Almond Shell Mulching in Avocado Organic Production. Soil Tillage Res. 2014, 143, 155–163. [Google Scholar] [CrossRef]
- Bonilla, N.; Vida, C.; Martínez-Alonso, M.; Landa, B.B.; Gaju, N.; Cazorla, F.M.; de Vicente, A. Organic Amendments to Avocado Crops Induce Suppressiveness and Influence the Composition and Activity of Soil Microbial Communities. Appl. Environ. Microbiol. 2015, 81, 3405–3418. [Google Scholar] [CrossRef]
- Vida, C.; Bonilla, N.; de Vicente, A.; Cazorla, F.M. Microbial Profiling of a Suppressiveness-Induced Agricultural Soil Amended with Composted Almond Shells. Front. Microbiol. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Peck, G.M.; Merwin, I.A.; Thies, J.E.; Schindelbeck, R.R.; Brown, M.G. Soil Properties Change during the Transition to Integrated and Organic Apple Production in a New York Orchard. Appl. Soil Ecol. 2011, 48, 18–30. [Google Scholar] [CrossRef]
- Walsh, B.D.; MacKenzie, A.F.; Salmins, S.; Buszard, D.J. Impact of Soil Management Systems on Organic Dwarf Apple Orchards and Soil Aggregate Stability, Bulk Density, Temperature and Water Content. Can. J. Soil Sci. 1996, 76, 203–209. [Google Scholar] [CrossRef]
- Baldi, E.; Toselli, M.; Marcolini, G.; Quartieri, M.; Cirillo, E.; Innocenti, A.; Marangoni, B. Compost Can Successfully Replace Mineral Fertilizers in the Nutrient Management of Commercial Peach Orchard. Soil Use Manag. 2010, 26, 346–353. [Google Scholar] [CrossRef]
- Hadas, A.; Kautsky, L.; Portnoy, R. Mineralization of Composted Manure and Microbial Dynamics in Soil as Affected by Long-Term Nitrogen Management. Soil Biol. Biochem. 1996, 28, 733–738. [Google Scholar] [CrossRef]
- Sanchez, J.E.; Willson, T.C.; Kizilkaya, K.; Parker, E.; Harwood, R.R. Enhancing the Mineralizable Nitrogen Pool through Substrate Diversity in Long Term Cropping Systems. Soil Sci. Soc. Am. J. 2001, 65, 1442–1447. [Google Scholar] [CrossRef]
- Stark, C.H.; Condron, L.M.; O’Callaghan, M.; Stewart, A.; Di, H.J. Differences in Soil Enzyme Activities, Microbial Community Structure and Short-Term Nitrogen Mineralisation Resulting from Farm Management History and Organic Matter Amendments. Soil Biol. Biochem. 2008, 40, 1352–1363. [Google Scholar] [CrossRef]
- Langmeier, M.; Frossard, E.; Kreuzer, M.; Mäder, P.; Dubois, D.; Oberson, A. Nitrogen Fertilizer Value of Cattle Manure Applied on Soils Originating from Organic and Conventional Farming Systems. Agronomy 2002, 22, 789–800. [Google Scholar] [CrossRef]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen Mineralization from Organic Amendments Is Variable but Predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef]
- Nett, L.; Ruppel, S.; Ruehlmann, J.; George, E.; Fink, M. Influence of Soil Amendment History on Decomposition of Recently Applied Organic Amendments. Soil Sci. Soc. Am. J. 2012, 76, 1290–1300. [Google Scholar] [CrossRef]
- Mallory, E.B.; Griffin, T.S. Impacts of Soil Amendment History on Nitrogen Availability from Manure and Fertilizer. Soil Sci. Soc. Am. J. 2007, 71, 964–973. [Google Scholar] [CrossRef]
- Schenker, M.B.; Pinkerton, K.E.; Mitchell, D.; Vallyathan, V.; Elvine-Kreis, B.; Green, F.H.Y. Pneumoconiosis from Agricultural Dust Exposure among Young California Farmworkers. Environ. Health Perspect. 2009, 117, 988–994. [Google Scholar] [CrossRef]
- Lepsch, H.C.; Brown, P.H.; Peterson, C.A.; Gaudin, A.C.M.; Khalsa, S.D.S. Impact of Organic Matter Amendments on Soil and Tree Water Status in a California Orchard. Agric. Water Manag. 2019, 222, 204–212. [Google Scholar] [CrossRef]
- Total Organic Carbon—Combustion Method. Available online: https://anlab.ucdavis.edu/analysis/Soils/322 (accessed on 2 May 2022).
- Total Nitrogen and Carbon—Combustion Method. Available online: https://anlab.ucdavis.edu/analysis/Soils/320 (accessed on 7 April 2022).
- Total Nitrogen and Carbon—Combustion Method. Available online: https://anlab.ucdavis.edu/analysis/Plant/522 (accessed on 2 May 2022).
- Horwath, W.R.; Paul, E.A. Microbial Biomass. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1994; pp. 753–773. ISBN 978-0-89118-865-0. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 1123–1184. ISBN 978-0-89118-866-7. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwath, W.R. Spectrophotometric Determination of Nitrate with a Single Reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia Determination Based on Indophenol Formation with Sodium Salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The Global Stoichiometry of Litter Nitrogen Mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Selles, F.; Wang, H.; Biederbeck, V.O.; Campbell, C.A. Carbon Dioxide Emissions and Transformation of Soil Carbon and Nitrogen during Wheat Straw Decomposition. Soil Sci. Soc. Am. J. 1998, 62, 1035–1041. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and Transformations along the Continuum from Litter to Soil Organic Matter in Forest Soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Schellenberg, D.L.; Alsina, M.M.; Muhammad, S.; Stockert, C.M.; Wolff, M.W.; Sanden, B.L.; Brown, P.H.; Smart, D.R. Yield-Scaled Global Warming Potential from N2O Emissions and CH4 Oxidation for Almond (Prunus Dulcis) Irrigated with Nitrogen Fertilizers on Arid Land. Agric. Ecosyst. Environ. 2012, 155, 7–15. [Google Scholar] [CrossRef]
- Volpiano, C.G.; Lisboa, B.B.; José, J.F.B.d.S.; Beneduzi, A.; Granada, C.E.; Vargas, L.K. Soil-Plant-Microbiota Interactions to Enhance Plant Growth. Rev. Bras. Ciênc. Solo 2022, 46, e0210098. [Google Scholar] [CrossRef]
- Haney, R.L.; Brinton, W.H.; Evans, E. Estimating Soil Carbon, Nitrogen, and Phosphorus Mineralization from Short-term Carbon Dioxide Respiration. Commun. Soil Sci. Plant Anal. 2008, 39, 2706–2720. [Google Scholar] [CrossRef]
- Grandy, A.S.; Neff, J.C. Molecular C Dynamics Downstream: The Biochemical Decomposition Sequence and Its Impact on Soil Organic Matter Structure and Function. Sci. Total Environ. 2008, 404, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Navarro, A.F.; Sánchez-Monedero, M.A.; Roig, A.; Cegarra, J. Influence of Sewage Sludge Compost Stability and Maturity on Carbon and Nitrogen Mineralization. Soil Biol. Biochem. 1998, 30, 305–313. [Google Scholar] [CrossRef]
- Deans, J.R.; Molina, J.A.E.; Clapp, C.E. Models for Predicting Potentially Mineralizable Nitrogen and Decomposition Rate Constants. Soil Sci. Soc. Am. J. 1986, 50, 323–326. [Google Scholar] [CrossRef]
- Franzluebbers, K.; Weaver, R.W.; Juo, A.S.R.; Franzluebbers, A.J. Mineralization of Carbon and Nitrogen from Cowpea Leaves Decomposing in Soils with Different Levels of Microbial Biomass. Biol. Fertil. Soils 1995, 19, 100–102. [Google Scholar] [CrossRef]
- Janzen, H.H. The Soil Carbon Dilemma: Shall We Hoard It or Use It? Soil Biol. Biochem. 2006, 38, 419–424. [Google Scholar] [CrossRef]
- Andrews, E.M.; Kassama, S.; Smith, E.E.; Brown, P.H.; Khalsa, S.D.S. A Review of Potassium-Rich Crop Residues Used as Organic Matter Amendments in Tree Crop Agroecosystems. Agriculture 2021, 11, 580. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Application of Eco-Physiological Quotients (qCO2 and qD) on Microbial Biomasses from Soils of Different Cropping Histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Wallenstein, M.D.; Schipanksi, M.E.; Grandy, A.S. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Sakamoto, K.; Oba, Y. Effect of Fungal to Bacterial Biomass Ratio on the Relationship between COz Evolution and Total Soil Microbial Biomass. Biol. Fertil. Soils 1994, 17, 39–44. [Google Scholar] [CrossRef]
- Spohn, M. Microbial Respiration per Unit Microbial Biomass Depends on Litter Layer Carbon-to-Nitrogen Ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Wardle, D.A.; Ghani, A. A Critique of the Microbial Metabolic Quotient (qCO2) as a Bioindicator of Disturbance and Ecosystem Development. Soil Biol. Biochem. 1995, 27, 1601–1610. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. The Metabolic Quotient for CO2 (qCO2) as a Specific Activity Parameter to Assess the Effects of Environmental Conditions, Such as pH, on the Microbial Biomass of Forest Soils. Soil Biol. Biochem. 1993, 23, 393–395. [Google Scholar] [CrossRef]
- Bonde, T.; Schnurer, J.; Rosswall, T. Microbial Biomass as a Fraction of Potentially Mineralizable Nitrogen in Soils from Long-Term Field Experiments. Soil Biol. Biochem. 1988, 20, 447–452. [Google Scholar] [CrossRef]
- Salazar-Villegas, A.; Blagodatskaya, E.; Dukes, J.S. Changes in the Size of the Active Microbial Pool Explain Short-Term Soil Respiratory Responses to Temperature and Moisture. Front. Microbiol. 2016, 7, 524. [Google Scholar] [CrossRef]


| SOCi | SOCf | TNi | TNf | ||
|---|---|---|---|---|---|
| g kg−1 | |||||
| Incubation | |||||
| Historic OMA | Hulls and Shells | 12.1 a | 13.1 a | 1.18 a | 1.43 a |
| Unamended | 12.1 a | 13.3 a | 1.18 a | 1.37 a | |
| No OMA | Hulls and Shells | 5.92 b | 5.18 b | 0.59 b | 0.58 b |
| Unamended | 5.92 b | 5.34 b | 0.59 b | 0.63 b | |
| Field | |||||
| Bulk soil | Hulls and Shells | 11.0 a | 10.8 a | 1.10 a | 1.13 a |
| Unamended | 11.0 a | 9.05 a | 1.10 a | 0.97 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartman, L.W.; Andrews, E.M.; Galatis, E.G.; Gaudin, A.C.M.; Brown, P.H.; Khalsa, S.D.S. Evaluation of Almond Hull and Shell Amendments across Organic Matter Management of Orchard Soils. Soil Syst. 2024, 8, 51. https://doi.org/10.3390/soilsystems8020051
Hartman LW, Andrews EM, Galatis EG, Gaudin ACM, Brown PH, Khalsa SDS. Evaluation of Almond Hull and Shell Amendments across Organic Matter Management of Orchard Soils. Soil Systems. 2024; 8(2):51. https://doi.org/10.3390/soilsystems8020051
Chicago/Turabian StyleHartman, Leah Wolff, Ellie M. Andrews, Erini G. Galatis, Amélie C. M. Gaudin, Patrick H. Brown, and Sat Darshan S. Khalsa. 2024. "Evaluation of Almond Hull and Shell Amendments across Organic Matter Management of Orchard Soils" Soil Systems 8, no. 2: 51. https://doi.org/10.3390/soilsystems8020051






