Separation of Boron and Arsenic from Geothermal Water with Novel Gel-Type Chelating Ion Exchange Resins: Batch and Column Sorption-Elution Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Batch Sorption Studies
2.2. Kinetics Studies
2.3. Batch Elution Tests
2.4. Column-Mode Sorption and Elution Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Batch-Mode Adsorption Tests
3.2.2. Kinetic Tests
3.2.3. Batch-Mode Elution Tests
3.2.4. Column-Mode Sorption and Elution Tests
3.2.5. Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Zeid, M.; Shiklomanov, I.A. Water Resources as a Challenge of the Twenty-First Century. Tenth IMO Lecture; World Meteorological Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Shiklomanov, I.A. Water in Crisis: A Guide to the World’s Fresh Water Resources; Oxford University Press: Oxford, UK, 1993; ISBN 0-19-507628-1. [Google Scholar]
- Jhansi, S.C.; Mishra, S.K. Wastewater Treatment and Reuse: Sustainability Options. Consilience 2013, 10, 1–15. [Google Scholar]
- U.S. Environmental Protection Agency. Basic Information about Water Reuse. Available online: https://www.epa.gov/waterreuse/basic-information-about-water-reuse (accessed on 13 October 2023).
- Englande, A.J.; Krenkel, P.; Shamas, J. Wastewater Treatment & Water Reclamation. Ref. Modul. Earth Syst. Environ. Sci. 2015, 639–670. [Google Scholar] [CrossRef]
- Baba, A. Application of Geothermal Energy and Its Environmental Problems in Turkey. Int. J. Glob. Environ. Issues 2015, 14, 321–331. [Google Scholar] [CrossRef]
- Richter, A. Mapping the Icelandic Geothermal Energy Sector; Íslandsstofa: Reykjavík, Iceland, 2016. [Google Scholar]
- Tomaszewska, B.; Rajca, M.; Kmiecik, E.; Bodzek, M.; Bujakowski, W.; Wątor, K.; Tyszer, M. The Influence of Selected Factors on the Effectiveness of Pre-Treatment of Geothermal Water during the Nanofiltration Process. Desalination 2017, 406, 74–82. [Google Scholar] [CrossRef]
- Melikoglu, M. Geothermal Energy in Turkey and around the World: A Review of the Literature and an Analysis Based on Turkey’s Vision 2023 Energy Targets. Renew. Sustain. Energy Rev. 2017, 76, 485–492. [Google Scholar] [CrossRef]
- Bundschuh, J.; Tomaszewska, B. Geothermal Water Management; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-317-56258-0. [Google Scholar]
- Shah, M.; Sircar, A.; Varsada, R.; Vaishnani, S.; Savaliya, U.; Faldu, M.; Vaidya, D.; Bhattacharya, P. Assessment of Geothermal Water Quality for Industrial and Irrigation Purposes in the Unai Geothermal Field, Gujarat, India. Groundw. Sustain. Dev. 2019, 8, 59–68. [Google Scholar] [CrossRef]
- Yoshizuka, K.; Kabay, N.; Bryjak, M. Arsenic and Boron in Geothermal Water and Their Removal. In The Global Arsenic Problem; CRC Press: Boca Raton, FL, USA, 2010; pp. 131–148. [Google Scholar]
- Bundschuh, J.; Maity, J.P.; Nath, B.; Baba, A.; Gunduz, O.; Kulp, T.R.; Jean, J.-S.; Kar, S.; Yang, H.-J.; Tseng, Y.-J.; et al. Naturally Occurring Arsenic in Terrestrial Geothermal Systems of Western Anatolia, Turkey: Potential Role in Contamination of Freshwater Resources. J. Hazard. Mater. 2013, 262, 951–959. [Google Scholar] [CrossRef]
- Bryjak, M.; Wolska, J.; Soroko, I.; Kabay, N. Adsorption-Membrane Filtration Process in Boron Removal from First Stage Seawater RO Permeate. Desalination 2009, 241, 127–132. [Google Scholar] [CrossRef]
- Yoshizuka, K.; Nishihama, S. Separation and Recovery of Boron From Various Resources Using Chelate Adsorbents. In Boron Separation Processes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 131–146. [Google Scholar]
- Belova, T.P.; Ershova, L.S. Boron Concentration by Industrial Anion Exchanger Resins from Model Solutions in a Dynamic Mode. Heliyon 2021, 7, e06141. [Google Scholar] [CrossRef]
- Figueira, M.; Reig, M.; Fernández de Labastida, M.; Cortina, J.L.; Valderrama, C. Boron Recovery from Desalination Seawater Brines by Selective Ion Exchange Resins. J. Environ. Manag. 2022, 314, 114984. [Google Scholar] [CrossRef]
- Hoang, V.A.; Nishihama, S.; Yoshizuka, K. Adsorptive Removal of Arsenic from Aqueous Environment. J. Chem. Eng. Jpn. 2019, 52, 829–834. [Google Scholar] [CrossRef]
- Xia, S.; Dong, B.; Zhang, Q.; Xu, B.; Gao, N.; Causseranda, C. Study of Arsenic Removal by Nanofiltration and Its Application in China. Desalination 2007, 204, 374–379. [Google Scholar] [CrossRef]
- Akin, I.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Removal of Arsenate [As(V)] and Arsenite [As(III)] from Water by SWHR and BW-30 Reverse Osmosis. Desalination 2011, 281, 88–92. [Google Scholar] [CrossRef]
- Urbano, B.F.; Rivas, B.L.; Martinez, F.; Alexandratos, S.D. Water-Insoluble Polymer-Clay Nanocomposite Ion Exchange Resin Based on N-Methyl-d-Glucamine Ligand Groups for Arsenic Removal. React. Funct. Polym. 2012, 72, 642–649. [Google Scholar] [CrossRef]
- Dambies, L.; Salinaro, R.; Alexandratos, S.D. Immobilized N-Methyl-d-Glucamine as an Arsenate-Selective Resin. Environ. Sci. Technol. 2004, 38, 6139–6146. [Google Scholar] [CrossRef]
- Cyganowski, P.; Şen, F.; Altıok, E.; Wolska, J.; Bryjak, M.; Kabay, N.; Arda, M.; Yüksel, M. Surface-Activated Chelating Resins Containing N-Methyl-d-Glucamine Functional Groups for Desalination of Geothermal Water Aimed for Removal of Boron and Arsenic. Solvent Extr. Ion Exch. 2021, 39, 584–603. [Google Scholar] [CrossRef]
- Samatya, S.; Tuncel, A.; Kabay, N. Boron removal from geothermal water by a novel monodisperse porous poly (GMA-co-EDM) resin containing N-methyl-d-glucamine functional group. Solvent Extr. Ion Exch. 2012, 30, 341–349. [Google Scholar] [CrossRef]
- Ozkula, G.; Urbano, B.F.; Rivas, B.L.; Kabay, N.; Bryjak, M. Arsenic sorption using mixtures of ion Exchange resins containing N-methyl-D-glucamine and quaternary ammonium groups. J. Chil. Chem. Soc. 2016, 61, 2752–2756. [Google Scholar] [CrossRef]
- Santander, P.; Rivas, B.L.; Urbano, B.F.; İpek, İ.Y.; Özkula, G.; Arda, M.; Yüksel, M.; Bryjak, M.; Kozlecki, T.; Kabay, N. Removal of boron from geothermal water by a novel boron selective resin. Desalination 2013, 310, 102–108. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Preparation of Polymeric Microspheres for Removal of Boron by Means of Sorption-Membrane Filtration Hybrid. Desalination 2011, 283, 193–197. [Google Scholar] [CrossRef]
- Ibezim-Ezeani, M.U.; Okoye, F.A.; Akaranta, O. Kinetic Studies on the Removal of Some Metal Ions from Aqueous Solution Using Modified Orange Mesocarp Extract. Int. J. Water Resour. Environ. Eng. 2012, 4, 192–200. [Google Scholar]
- Hameed, B.H.; Rahman, A.A. Removal of Phenol from Aqueous Solutions by Adsorption onto Activated Carbon Prepared from Biomass Material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
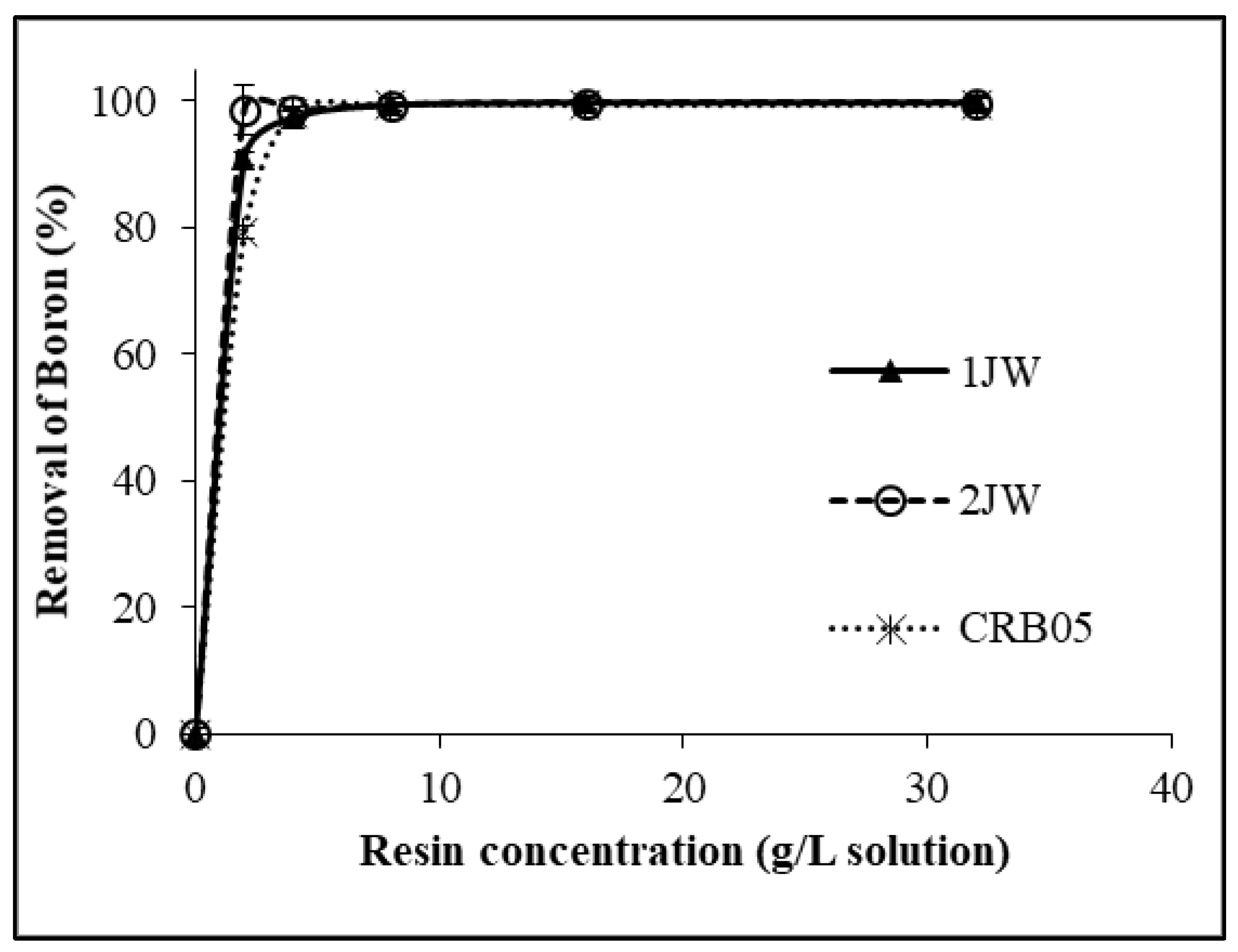




| Amount of Adsorbent (g Resin/L Solution) | The Concentration of Boron in the Solution after Sorption (mg/L) | ||
|---|---|---|---|
| 1JW | 2JW | Diaion CRB05 | |
| 0 | 10.06 | 10.06 | 10.06 |
| 2 | 0.87 | 0.13 | 1.97 |
| 4 | 0.26 | 0.03 | 0.18 |
| 8 | 0.07 | 0.03 | 0.06 |
| 16 | 0.03 | 0.03 | 0.05 |
| 32 | 0.03 | 0.02 | 0.05 |
| Amount of Adsorbent (g Resin/L Solution) | The Concentration of Arsenic in the Solution after Sorption (mg/L) | ||
|---|---|---|---|
| 1JW | 2JW | Diaion CRB05 | |
| 0 | 0.160 | 0.160 | 0.160 |
| 2 | 0.075 | 0.070 | 0.121 |
| 4 | 0.026 | 0.019 | 0.069 |
| 8 | 0.008 | 0.009 | 0.040 |
| 16 | 0.006 | 0.006 | 0.020 |
| 32 | 0.004 | 0.006 | 0.009 |
| Resin | Feed Boron Concentration (mg/L) | Resin Amount (g/L) | Removal (%) | Reference |
|---|---|---|---|---|
| 1JW | 10.06 | 2 | 91.0 | This Study |
| 2JW | 10.06 | 2 | 98.6 | This Study |
| CRB05 | 10.06 | 4 | 98.7 | This Study |
| 1PTN | 10.90 | 32 | 64.0 | [23] |
| 2PTN | 10.90 | 16 | 96.0 | [23] |
| poly(GMA-co-EDM) containing NMDG | 11.00 | 4 | 94.0 | [24] |
| 37Diaion CRB05 | 4.43 | 2 | 90.0 | [25] |
| Diaion CRB02 | 10.50 | 3.2 | 85.0 | [26] |
| P(VbNMDG) | 10.50 | 1.6 | 98.0 | [26] |
| Resin | Feed Arsenic Concentration (mg/L) | Resin Amount (g/L) | Removal (%) | |
| 1JW | 0.160 | 8 | 94.8 | This Study |
| 2JW | 0.160 | 8 | 94.1 | This Study |
| Diaion CRB05 | 0.160 | 32 | 94.0 | This Study |
| 1PTN | 0.160 | 32 | 18.0 | [23] |
| 2PTN | 0.160 | 16 | 93.0 | [27] |
| PVBNMDG | 10.0 | 5 | 95.0 | [21] |
| NMDG-DMA | 1.00 | 1.6 | 95.2 | [22] |
| Time (min) | The Concentration of Boron in the Solution after Sorption (mg/L) | ||
|---|---|---|---|
| 1JW | 2JW | Diaion CRB05 | |
| 0 | 8.4 | 9.5 | 9.9 |
| 5 | 5.2 | 4.2 | 7.2 |
| 10 | 4.6 | 4.1 | 0.6 |
| 15 | 3.6 | 3.3 | 0.5 |
| 30 | 2.3 | 2.1 | 0.5 |
| 60 | 1.6 | 1.2 | 0.2 |
| 120 | 0.6 | 1.0 | 0.1 |
| 240 | 0.5 | 0.4 | 0.1 |
| 360 | 0.2 | 0.3 | 0.1 |
| 480 | 0.7 | 0.3 | 0.1 |
| 1440 | 1.1 | 0.2 | 0.1 |
| Model * | Equation ** | Rate Determination Step |
|---|---|---|
| ISV | F(X) = −ln(1 − X) = K1i where K1i = 3DC/roδCr | Film Diffusion |
| ISV | F(X) = −ln(1 − X2) = kt where k = Drπ2/ro2 | Particle Diffusion |
| UC | F(X) = X = (3CAoKmA/aroCso)t | Liquid Film |
| UC | F(X) = 3−3(1 − X)2/3-2X = (6DeRCAo/aro2Cso)t | Reacted Layer |
| UC | F(X) = 1− (1 − X)1/3 = (ksCAo/aroCo)t | Chemical Reaction |
| Resins | Conventional Kinetic Modeling | Diffusional and Reaction Models | |||||
|---|---|---|---|---|---|---|---|
| Infinite Solution Volume | Unreacted Core Model | ||||||
| Pseudo 1st Order | Pseudo 2nd Order | Film Diffusion | Particle Diffusion | Liquid Film | Reacted Layer | Chemical Reaction | |
| 1JW | R2: 0.98 | R2: 0.99 | R2: 0.96 | R2: 0.99 | R2: 0.64 | R2: 0.92 | R2: 0.98 |
| qe: 0.97 | qe: 0.19 | ||||||
| k1: 0.41 | k2: 55.2 | ||||||
| 2JW | R2: 0.81 | R2: 0.99 | R2: 0.96 | R2: 0.99 | R2: 0.46 | R2: 0.70 | R2: 0.82 |
| qe: 0.98 | qe: 0.83 | ||||||
| k1: 0.80 | k2: 6.55 | ||||||
| Diaion CRB 05 | R2: 0.80 | R2: 0.99 | R2: 0.96 | R2: 0.99 | R2: 0.95 | R2: 0.78 | R2: 0.85 |
| qe: 0.59 | qe: 0.68 | ||||||
| k1: 1.62 | k2: 5.32 | ||||||
| SORPTION | ELUTION | |||||
|---|---|---|---|---|---|---|
| Sorption Feed | Eluent | Resin | maft_s (mg) | Boron Removal (%) | maft_el (mg) | Elution Efficiency (%) |
| MBAS | 0.5 M H2SO4 | 1JW | 0.02 | 91.7 | 0.18 | 74.1 |
| 2JW | 0.02 | 91.7 | 0.23 | 93.6 | ||
| CRB 05 | 0.03 | 89.7 | 0.24 | 99.0 | ||
| 1 M H2SO4 | 1JW | 0.03 | 89.2 | 0.23 | 99.5 | |
| 2JW | 0.02 | 90.6 | 0.22 | 96.8 | ||
| CRB 05 | 0.02 | 91.3 | 0.23 | 99.0 | ||
| GW | 1 M H2SO4 | 1JW | 0.04 | 83.38 | 0.21 | 97.7 |
| 2JW | 0.01 | 94.29 | 0.20 | 99.8 | ||
| CRB 05 | 0.02 | 93.30 | 0.24 | 99.8 | ||
| The Data Calculated from the Breakthrough Curves | 1JW | 2JW | Diaion CRB05 |
|---|---|---|---|
| Breakthrough capacity (mg B) | 1.76 | 0.91 | 1.99 |
| The breakthrough capacity of resin (mg/mL resin) | 3.52 | 1.82 | 3.98 |
| Total capacity (mg B) | 2.54 | 2.32 | 3.15 |
| The total exchange capacity of resin (mg B/mL resin) | 5.08 | 4.64 | 6.29 |
| Degree of column utilization (%) | 69.3 | 39.4 | 63.1 |
| Total eluted boron (mg B) | 1.86 | 2.22 | 2.85 |
| Elution efficiency (%) | 73.1 | 95.7 | 90.4 |
| Parameters | 1JW | 2JW |
|---|---|---|
| Resin type | Gel | Expanded gel |
| Composition | VBC/S/DVB | VBC/DVB |
| Water content (%) | 42 | 78 |
| IEC (mmol/g) | 2.1 | 2.3 |
| Functional group | (R-N(CH3)-C6H8(OH)5) | |
| Parameters | Diaion CRB 05 |
|---|---|
| Resin type | Chelating resin |
| Composition | S/DVB |
| Water content (%) | 43–53 |
| IEC (mmol/g) | 0.95 |
| Functional group | (R-N(CH3)-C6H8(OH)5) |
| Specifications | GW | Specifications | GW |
|---|---|---|---|
| pH | 8.40 | Ca2+ (mg/L) | 29.76 |
| EC (µS/cm) | 1721 | Mg2+ (mg/L) | 7.11 |
| TDS (mg/L) | 863 | F− (mg/L) | 6.34 |
| Salinity (‰) | 0.87 | Cl− (mg/L) | 209.67 |
| (HCO3)− (mg/L) | 655 | NO3− (mg/L) | 2.22 |
| Li+ (mg/L) | 1.24 | SO42− (mg/L) | 161.6 |
| Na+ (mg/L) | 309.6 | B (mg/L) | 10.94 |
| K+ (mg/L) | 27.9 | SiO2 (mg/L) | 118.9 |
| NH4+ (mg/L) | 2.94 | As (mg/L) | 0.160 |
| Resin | 1JW | 2JW | Diaion CRB 05 |
|---|---|---|---|
| Resin amount (g resin/L solution) | 4.00 | 4.00 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altıok, E.; Şen, F.; Wolska, J.; Cyganowski, P.; Bryjak, M.; Kabay, N.; Arda, M.; Yüksel, M. Separation of Boron and Arsenic from Geothermal Water with Novel Gel-Type Chelating Ion Exchange Resins: Batch and Column Sorption-Elution Studies. Molecules 2023, 28, 7708. https://doi.org/10.3390/molecules28237708
Altıok E, Şen F, Wolska J, Cyganowski P, Bryjak M, Kabay N, Arda M, Yüksel M. Separation of Boron and Arsenic from Geothermal Water with Novel Gel-Type Chelating Ion Exchange Resins: Batch and Column Sorption-Elution Studies. Molecules. 2023; 28(23):7708. https://doi.org/10.3390/molecules28237708
Chicago/Turabian StyleAltıok, Esra, Fatma Şen, Joanna Wolska, Piotr Cyganowski, Marek Bryjak, Nalan Kabay, Müşerref Arda, and Mithat Yüksel. 2023. "Separation of Boron and Arsenic from Geothermal Water with Novel Gel-Type Chelating Ion Exchange Resins: Batch and Column Sorption-Elution Studies" Molecules 28, no. 23: 7708. https://doi.org/10.3390/molecules28237708






