PPy-Coated Mo3S4/CoMo2S4 Nanotube-like Heterostructure for High-Performance Lithium Storage
Abstract
:1. Introduction
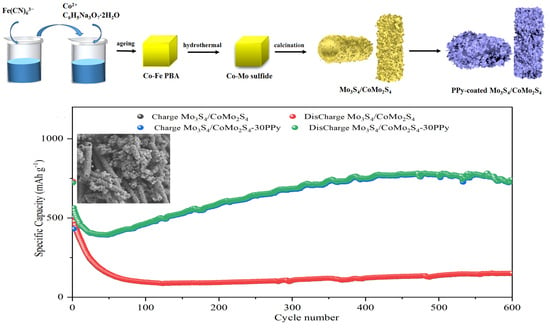
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Co-Fe Prussian Blue (Co-Fe PBA)
3.3. Preparation of Mo3S4/CoMo2S4 Heterostructure
3.4. Preparation of PPy-Coated Mo3S4/CoMo2S4 Heterostructure
3.5. Characterization
3.6. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, D.; Shamim, N.; Crawford, A.; Huang, Q.; Vartanian, C.K.; Viswanathan, V.V.; Paiss, M.D.; Alam, M.J.E.; Reed, D.M.; Sprenkle, V.L. Li-ion battery technology for grid application. J. Power Sources 2021, 511, 230419. [Google Scholar] [CrossRef]
- Demirocak, D.; Srinivasan, S.; Stefanakos, E. A Review on Nanocomposite Materials for Rechargeable Li-ion Batteries. Appl. Sci. 2017, 7, 731. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Li, X.; Liu, Z.; Sun, J.; Gao, S. Amorphous carbon and carbon nanotubes synergistically reinforced with MnO2 as a cathode material for zinc-ion batteries. Diam. Relat. Mater. 2023, 132, 109615. [Google Scholar] [CrossRef]
- Miao, Z.; Gao, K.; Li, D.; Gao, Z.; Zhao, W.; Li, Z.; Sun, W.; Wang, X.; Zhang, H.; Wang, X.; et al. Heterointerface Engineered Core-Shell Fe2O3@TiO2 for High-Performance Lithium-Ion Storage. Molecules 2023, 28, 6903. [Google Scholar] [CrossRef] [PubMed]
- Chchiyai, Z.; El Ghali, O.; Lahmar, A.; Alami, J.; Manoun, B. Design and Performance of a New Zn0.5Mg0.5FeMnO4 Porous Spinel as Anode Material for Li-Ion Batteries. Molecules 2023, 28, 7010. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, Z.; Yang, L.; Liu, J.; Xu, Y.; Li, G. Facile synthesis of sulfur doped Sb2Se3 nanosheets with enhanced electrochemical performance. J. Alloys Compd. 2013, 579, 209–217. [Google Scholar] [CrossRef]
- Roy, K.; Banerjee, A.; Ogale, S. Search for New Anode Materials for High Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 20326–20348. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Hou, B.; Pawar, S.M.; Kim, H.; Im, H. Graphene-integrated CuCo2S4 microspheres as a sustainable anode material for high-performance Li-ion batteries. Int. J. Energy Res. 2020, 45, 1613–1626. [Google Scholar] [CrossRef]
- Cui, Z.; Mei, T.; Yao, J.; Hou, B.; Zhu, X.; Liu, X.; Wang, X. Cabbage-like nitrogen-doped graphene/sulfur composite for lithium-sulfur batteries with enhanced rate performance. J. Alloys Compd. 2018, 753, 622–629. [Google Scholar] [CrossRef]
- Gao, Y.P.; Huang, K.J. NiCo2S4 Materials for Supercapacitor Applications. Chem. Asian J. 2017, 12, 1969–1984. [Google Scholar] [CrossRef]
- Han, D.; Wei, J.; Zhao, Y.; Shen, Y.; Pan, Y.; Wei, Y.; Mao, L. Metal–organic framework derived petal-like Co3O4@CoNi2S4 hybrid on carbon cloth with enhanced performance for supercapacitors. Inorg. Chem. Front. 2020, 7, 1428–1436. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, J.; He, Y.; Li, J.; Xu, J.; Sun, Y.; Liao, J.; Zhou, X. Construction of CoS2 nanoparticles embedded in well-structured carbon nanocubes for high-performance potassium-ion half/full batteries. Sci. China Chem. 2021, 64, 1401–1409. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The application of nanostructured transition metal sulfides as anodes for lithium ion batteries. J. Energy Chem. 2018, 27, 1536–1554. [Google Scholar] [CrossRef]
- Hou, Z.; Jiang, M.; Cao, Y.; Liu, H.; Zhang, Y.; Wang, J.-G. Encapsulating ultrafine cobalt sulfides into multichannel carbon nanofibers for superior Li-ion energy storage. J. Power Sources 2022, 541, 231682. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Yang, C.; Wang, Y.; Gu, S.; Zhou, G. Core–Shell Structure Trimetallic Sulfide@N-Doped Carbon Composites as Anodes for Enhanced Lithium-Ion Storage Performance. Molecules 2023, 28, 7580. [Google Scholar] [CrossRef]
- Liu, C.; Fang, X.; Peng, H.; Li, Y.; Yang, Y. Fabrication of Composite Gel Electrolyte and F-Doping Carbon/Silica Anode from Electro-Spun P(VDF-HFP)/Silica Composite Nanofiber Film for Advanced Lithium-Ion Batteries. Molecules 2023, 28, 5304. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Li, T.; Liang, L.; Wen, S.; Zhang, Y.; Liu, G.; Ren, F.; Wang, G. Efficient Regulation of Polysulfides by Anatase/Bronze TiO2 Heterostructure/Polypyrrole Composites for High-Performance Lithium-Sulfur Batteries. Molecules 2023, 28, 4286. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Bresser, D.; Ji, Y.; Geiger, D.; Kaiser, U.; Streb, C.; Varzi, A.; Passerini, S. Cobalt Disulfide Nanoparticles Embedded in Porous Carbonaceous Micro-Polyhedrons Interlinked by Carbon Nanotubes for Superior Lithium and Sodium Storage. ACS Nano 2018, 12, 7220–7231. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Han, L.; Liao, C.; Mu, X.; Kan, Y.; Hu, Y. Polyacrylonitrile@metal organic frameworks composite-derived heteroatoms doped carbon@encapsulated cobalt sulfide as superb sodium ion batteries anode. J. Colloid Interface Sci. 2021, 581, 552–565. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.-Y.; Belharouak, I.; Sun, Y.-K. Superior Li/Na-storage capability of a carbon-free hierarchical CoSx hollow nanostructure. Nano Energy 2017, 32, 320–328. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Self-Assembled Interwoven Nanohierarchitectures of NaNbO3 and NaNb1–xTaxO3 (0.05 ≤ x ≤ 0.20): Synthesis, Structural Characterization, Photocatalytic Applications, and Dielectric Properties. ACS Omega 2022, 7, 16952–16967. [Google Scholar] [CrossRef]
- Farooq, U.; Chaudhary, P.; Ingole, P.P.; Kalam, A.; Ahmad, T. Development of Cuboidal KNbO3@α-Fe2O3 Hybrid Nanostructures for Improved Photocatalytic and Photoelectrocatalytic Applications. ACS Omega 2020, 5, 20491–20505. [Google Scholar] [CrossRef]
- Farooq, U.; Naz, F.; Phul, R.; Pandit, N.A.; Jain, S.K.; Ahmad, T. Development of Heterostructured Ferroelectric SrZrO3/CdS Photocatalysts with Enhanced Surface Area and Photocatalytic Activity. J. Nanosci. Nanotechnol. 2020, 20, 3770–3779. [Google Scholar] [CrossRef]
- Naaz, F.; Ahmad, T. Ag-Doped WO3 Nanoplates as Heterogenous Multifunctional Catalyst for Glycerol Acetylation, Electrocatalytic and Enhanced Photocatalytic Hydrogen Production. Langmuir 2023, 39, 9300–9314. [Google Scholar] [CrossRef]
- Naaz, F.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Unraveling the chemoselective catalytic, photocatalytic and electrocatalytic applications of copper supported WO3 nanosheets. Catal. Commun. 2023, 178, 106678. [Google Scholar] [CrossRef]
- Zheng, J.; He, C.; Li, X.; Wang, K.; Wang, T.; Zhang, R.; Tang, B.; Rui, Y. CoS2–MnS@Carbon nanoparticles derived from metal–organic framework as a promising anode for lithium-ion batteries. J. Alloys Compd. 2021, 854, 157315. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Qiu, Y.; Guo, Z.; Liu, H.; Wang, P.; Tian, S.; Ding, H.; Du, B.; Qian, L. In situ F doping-induced multilayer FeS2@CoS@C hierarchical heterostructures for ultrafast lithium storage. Mater. Today Energy 2022, 29, 101108. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Chen, H.; Guo, X.; Zhang, Z.; Liu, J.; Mao, C.; Li, G. Constructing heterostructured FeS2/CuS nanospheres as high rate performance lithium ion battery anodes. Inorg. Chem. Front. 2020, 7, 1900–1908. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Yang, G.; Cai, F.; Li, G. Synthesis of Honeycomb-Like Co3S4/MoS2 Composites with Hollow Structure As Anode Materials for High-Performance Lithium-Ion and Sodium-Ion Batteries. J. Electron. Mater. 2020, 49, 6519–6527. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, M.; Qian, F.; Zeng, P.; Chen, M.; Wang, R.; Liu, Y.; Ding, Y.; Fang, Z. Heterostructure CoS/NC@MoS2 hollow spheres for high-performance hydrogen evolution reactions and lithium-ion batteries. ChemElectroChem 2018, 5, 3953–3960. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Diao, Y.; Wei, L.; Chen, J.; Wang, F. CoMo2S4 with Superior Conductivity for Electrocatalytic Hydrogen Evolution: Elucidating the Key Role of Co. Adv. Funct. Mater. 2021, 31, 2103732. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Zan, P.; Zhao, L.; Lian, J. Growth of vertically aligned Co3S4/CoMo2S4 ultrathin nanosheets on reduced graphene oxide as a high-performance supercapacitor electrode. J. Mater. Chem. A 2016, 4, 18857–18867. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Yuan, H.; Wu, X.; Zhang, D. Synthesis of C/MoS2-CoMo2S4 for application in Li-O2 batteries. Electrochim. Acta 2022, 409, 139790. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Henzie, J.; Jiang, B.; Xia, W.; Chen, T.; Bando, Y.; Kang, Y.-M.; Hossain, M.S.A.; Sugahara, Y.; et al. Mesoporous Iron-doped MoS2/CoMo2S4 Heterostructures through Organic–Metal Cooperative Interactions on Spherical Micelles for Electrochemical Water Splitting. ACS Nano 2020, 14, 4141–4152. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Zhang, L.; Zhang, Y.; Li, Y.; Xu, X.; Lin, K.; Du, Y. In-situ construction of Mo3S4/Cd0.5Zn0.5S heterojunction: An efficient and stable photocatalyst for H2 evolution. Int. J. Hydrogen Energy 2020, 45, 21014–21023. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Wang, Z.; Kang, Y.-M.; Bando, Y.; Yamauchi, Y. Elaborately assembled core-shell structured metal sulfides as a bifunctional catalyst for highly efficient electrochemical overall water splitting. Nano Energy 2018, 47, 494–502. [Google Scholar] [CrossRef]
- Huang, Z.X.; Wang, Y.; Wong, J.I.; Shi, W.H.; Yang, H.Y. Synthesis of self-assembled cobalt sulphide coated carbon nanotube and its superior electrochemical performance as anodes for Li-ion batteries. Electrochim. Acta 2015, 167, 388–395. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, P.; Zheng, X.; Cao, J.; Liu, Y.; Feng, J.; Qi, J. Constructing MoS2/CoMo2S4/Co3S4 nanostructures supported by graphene layers as the anode for lithium-ion batteries. Dalton Trans. 2020, 49, 1167–1172. [Google Scholar] [CrossRef]
- Lu, Y.; Fong, E. Biomass-mediated synthesis of carbon-supported nanostructured metal sulfides for ultra-high performance lithium-ion batteries. J. Mater. Chem. A 2016, 4, 2738–2745. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Huang, S.; Zhang, Y.; Chen, Z. Integrated design of sandwich-like C@MoS2@C nanospheres as active anode material for lithium-ion batteries. J. Mater. Sci. 2022, 57, 14948–14958. [Google Scholar] [CrossRef]
- Dominguez, N.; Torres, B.; Barrera, L.A.; Rincon, J.E.; Lin, Y.; Chianelli, R.R.; Ahsan, M.A.; Noveron, J.C. Bimetallic CoMoS Composite Anchored to Biocarbon Fibers as a High-Capacity Anode for Li-Ion Batteries. ACS Omega 2018, 3, 10243–10249. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, C.; Zhong, Y.; Chen, M.; Cai, L.; Wang, H.; Liu, X.; Cao, G.; Li, W. Highly dispersed Co-Mo sulfide nanoparticles on reduced graphene oxide for lithium and sodium ion storage. Nano Res. 2020, 13, 188–195. [Google Scholar] [CrossRef]
- Lu, B.; Liu, J.; Hu, R.; Wang, H.; Liu, J.; Zhu, M. C@MoS2@PPy sandwich-like nanotube arrays as an ultrastable and high-rate flexible anode for Li/Na-ion batteries. Energy Stor. Mater. 2018, 14, 118–128. [Google Scholar] [CrossRef]
- Xie, D.; Wang, D.H.; Tang, W.J.; Xia, X.H.; Zhang, Y.J.; Wang, X.L.; Gu, C.D.; Tu, J.P. Binder-free network-enabled MoS2-PPY-rGO ternary electrode for high capacity and excellent stability of lithium storage. J. Power Sources 2016, 307, 510–518. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, Y.; Su, Y.; Zhai, W.; Xu, S. MoS2/SnS/CoS Heterostructures on Graphene: Lattice-Confinement Synthesis and Boosted Sodium Storage. Molecules 2023, 28, 5972. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Sun, J.; Liu, Y.; Gao, L.; Yao, H.; Lin, C. Heat-induced formation of porous and free-standing MoS2/GS hybrid electrodes for binder-free and ultralong-life lithium ion batteries. Nano Energy 2014, 8, 183–195. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Synthesis of MoS2 nanosheet–graphene nanosheet hybrid materials for stable lithium storage. ChemComm 2013, 49, 1838. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, A.; Zhu, Y.; Zhou, S.; Duan, Y.; Tang, Q.; Deng, W.; Chen, X. Hierarchical microstructure of CNTs interwoven ultrathin Co3S4 nanosheets as a high performance anode for sodium-ion battery. Ceram. Int. 2019, 45, 3591–3599. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, F.; Jiang, W.; Xie, J.; Zhao, D.; Meng, Y.; Yang, Z.; Lv, Z.; Xu, Y.; Sun, W.; Jiang, Z. PPy-Coated Mo3S4/CoMo2S4 Nanotube-like Heterostructure for High-Performance Lithium Storage. Molecules 2024, 29, 234. https://doi.org/10.3390/molecules29010234
Tang F, Jiang W, Xie J, Zhao D, Meng Y, Yang Z, Lv Z, Xu Y, Sun W, Jiang Z. PPy-Coated Mo3S4/CoMo2S4 Nanotube-like Heterostructure for High-Performance Lithium Storage. Molecules. 2024; 29(1):234. https://doi.org/10.3390/molecules29010234
Chicago/Turabian StyleTang, Fei, Wei Jiang, Jingjing Xie, Deyang Zhao, Yanfeng Meng, Zhenglong Yang, Zhiqiang Lv, Yanbin Xu, Wenjuan Sun, and Ziqiao Jiang. 2024. "PPy-Coated Mo3S4/CoMo2S4 Nanotube-like Heterostructure for High-Performance Lithium Storage" Molecules 29, no. 1: 234. https://doi.org/10.3390/molecules29010234