A Comprehensive Study to Determine the Residual Elimination Pattern of Major Metabolites of Amoxicillin–Sulbactam Hybrid Molecules in Rats by UPLC–MS/MS
Abstract
:1. Introduction
2. Results
2.1. Method Validation
2.1.1. Specificity
2.1.2. Limits of Detection (LODs) and Quantitation (LOQs)
2.1.3. Linearity
2.1.4. Recovery and Precision
2.2. Residual Elimination Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Chemicals and Reagents
4.1.2. Instruments
4.1.3. Solution Preparation
4.2. Detection Methods for Major Metabolites of AS
4.2.1. UPLC–MS/MS for Major Metabolites of AS
UPLC–MS/MS Instrumental Conditions for AMO, AMA, DIKETO, and AMP
UPLC–MS/MS Instrumental Conditions for SBT
4.2.2. Limits of Detection (LODs) and Quantitation (LOQs)
4.2.3. Linearity
4.2.4. Recovery and Precision
4.3. Residual Elimination of Major AS Metabolites in Rats
4.3.1. Experimental Design and Groups
4.3.2. Sample Collection
4.3.3. Determination of Sample Concentrations
4.3.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A





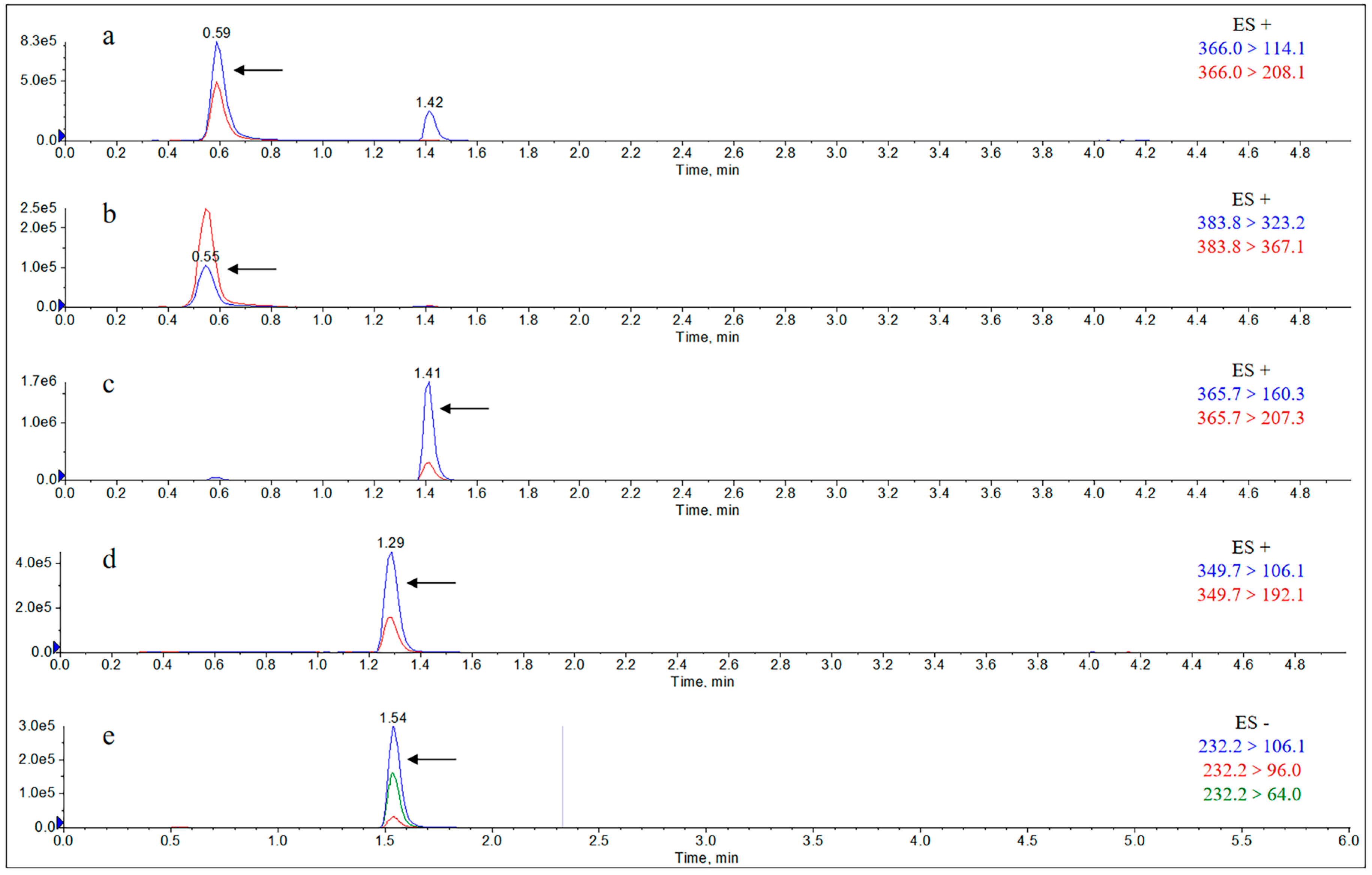





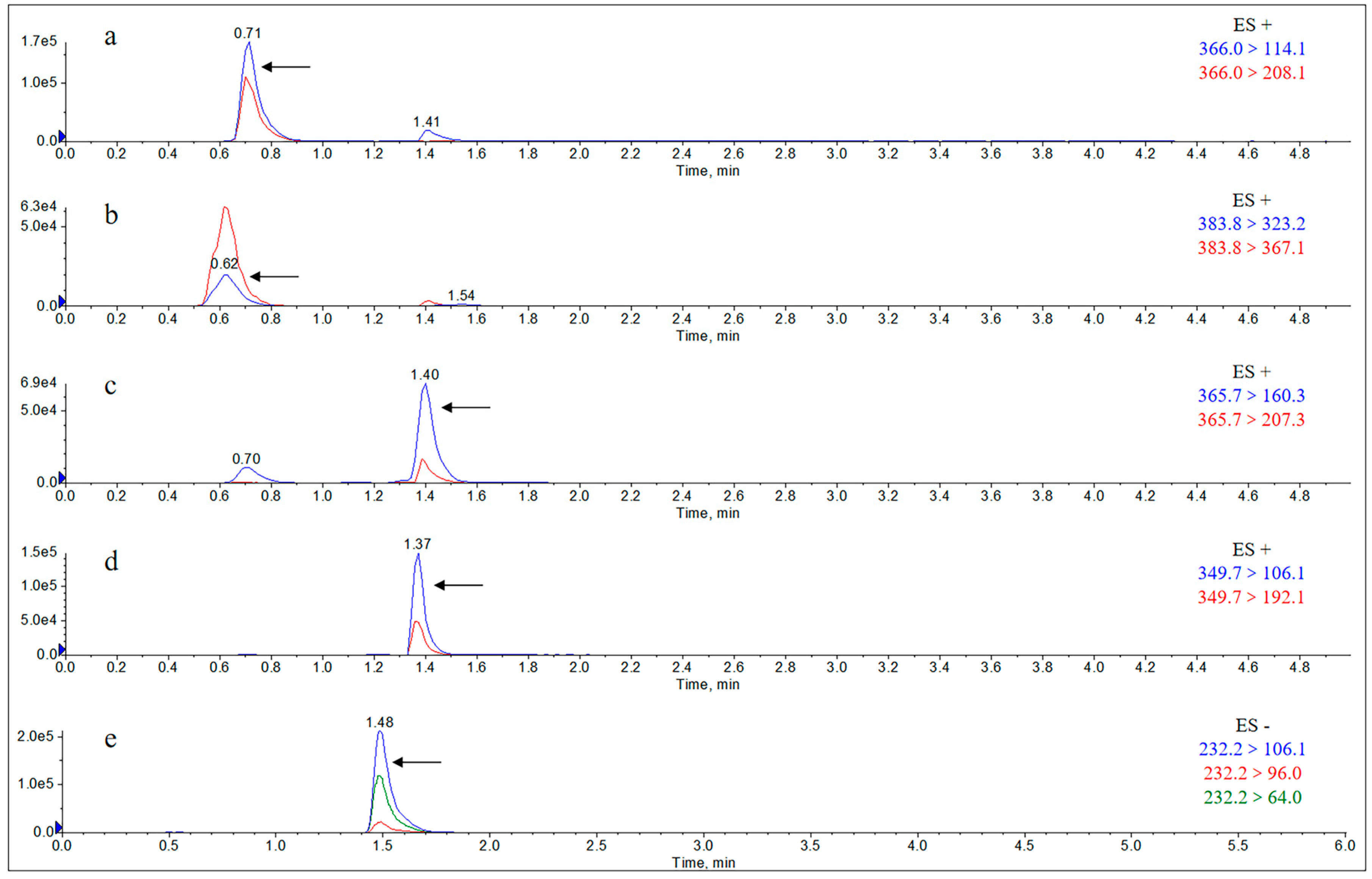

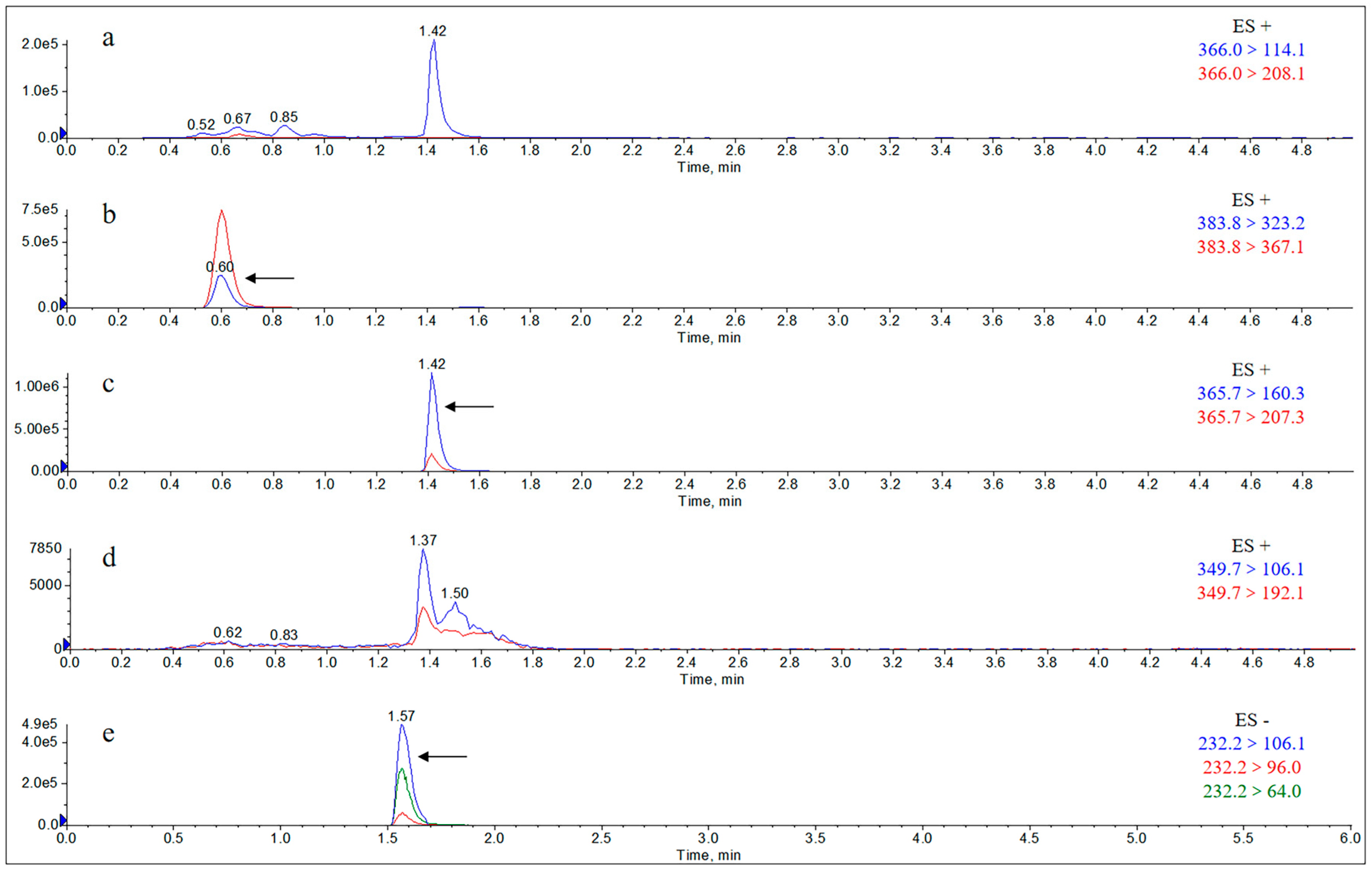


Appendix B
| Matrix | Batch | Linearity Range (μg/mL or μg/g) | Linear Equation | Correlation Coefficient |
|---|---|---|---|---|
| Urine | 1 | 0.025~10 | y = 0.5629x − 0.0351 | 0.9994 |
| 2 | 0.025~10 | y = 0.4438x + 0.0378 | 0.9985 | |
| 3 | 0.025~10 | y = 0.4947x − 0.0348 | 0.9996 | |
| Liver | 1 | 0.025~10 | y = 0.7677x − 0.0668 | 0.9991 |
| 2 | 0.025~10 | y = 0.6209x − 0.0507 | 0.9993 | |
| 3 | 0.025~10 | y = 0.6152x + 0.0300 | 0.9948 | |
| Kidney | 1 | 0.025~10 | y = 0.6462x − 0.0194 | 0.9997 |
| 2 | 0.025~10 | y = 0.5698x + 0.0220 | 0.9994 | |
| 3 | 0.025~10 | y = 0.5861x + 0.0335 | 0.9992 |
| Matrix | Batch | Linearity Range (μg/mL or μg/g) | Linear Equation | Correlation Coefficient |
|---|---|---|---|---|
| Urine | 1 | 0.025~10 | y = 0.1804x + 0.0009 | 0.9994 |
| 2 | 0.025~10 | y = 0.2103x + 0.0422 | 0.9991 | |
| 3 | 0.025~10 | y = 0.1782x + 0.0163 | 0.9992 | |
| Liver | 1 | 0.025~10 | y = 0.2665x + 0.0334 | 0.9987 |
| 2 | 0.025~10 | y = 0.2204x + 0.0181 | 0.9994 | |
| 3 | 0.025~10 | y = 0.2165x + 0.0097 | 0.9995 | |
| Kidney | 1 | 0.025~10 | y = 0.2541x + 0.0036 | 0.9992 |
| 2 | 0.025~10 | y = 0.1845x + 0.0147 | 0.9991 | |
| 3 | 0.025~10 | y = 0.2306x − 0.0136 | 0.9996 | |
| Feces | 1 | 0.05~10 | s = 317,516c + 36,850 | 0.9985 |
| 2 | 0.05~10 | s = 329,082c + 40,839 | 0.9938 | |
| 3 | 0.05~10 | s = 339,320c + 51,023 | 0.9929 |
| Matrix | Batch | Linearity Range (μg/mL or μg/g) | Linear Equation | Correlation Coefficient |
|---|---|---|---|---|
| Urine | 1 | 0.001~10 | y = 1.0708x − 0.0057 | 0.9993 |
| 2 | 0.001~10 | y = 0.8586x − 0.0583 | 0.9982 | |
| 3 | 0.001~10 | y = 0.8617x + 0.0322 | 0.9975 | |
| Liver | 1 | 0.001~10 | y = 0.7537x − 0.0916 | 0.9992 |
| 2 | 0.001~10 | y = 0.7073x − 0.0755 | 0.9935 | |
| 3 | 0.001~10 | y = 0.7404x − 0.0651 | 0.9994 | |
| Kidney | 1 | 0.001~10 | y = 0.6684x − 0.0611 | 0.9991 |
| 2 | 0.001~10 | y = 0.7204x − 0.0601 | 0.9995 | |
| 3 | 0.001~10 | y = 0.7515x + 0.0029 | 0.9942 | |
| Feces | 1 | 0.0025~10 | s = 429,253c − 15,595 | 0.9992 |
| 2 | 0.0025~10 | s = 330,080c + 9065.5 | 0.9962 | |
| 3 | 0.0025~10 | s = 343,413c + 16,205 | 0.9957 |
| Matrix | Batch | Linearity Range (μg/mL or μg/g) | Linear Equation | Correlation Coefficient |
|---|---|---|---|---|
| Urine | 1 | 0.025~10 | s = 125,656c + 5734.3 | 0.9988 |
| 2 | 0.025~10 | s = 142,231c + 4139.1 | 0.9992 | |
| 3 | 0.025~10 | s = 128,916c + 9636.3 | 0.9976 | |
| Liver | 1 | 0.025~10 | s = 608,799c + 20,531 | 0.9993 |
| 2 | 0.025~10 | s = 525,809c −13,796 | 0.9995 | |
| 3 | 0.025~10 | s = 501,645c + 16,491 | 0.9983 | |
| Kidney | 1 | 0.025~10 | s = 649,630c + 40,793 | 0.9963 |
| 2 | 0.025~10 | s = 532,714c + 15,408 | 0.9992 | |
| 3 | 0.025~10 | s = 711,610c + 1353.9 | 0.9956 | |
| Feces | 1 | 0.05~10 | s = 610,688c − 44,116 | 0.9942 |
| 2 | 0.05~10 | s = 667,957c − 65,813 | 0.9994 | |
| 3 | 0.05~10 | s = 755,649c + 13,200 | 0.9995 |
| Matrix | Added Concentration (μg/mL or μg/g) | Recovery Rate ( ± S.D., %, n = 5) | Intra-Batch Precision (%, n = 5) | Inter-Batch Precision (%, n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| Urine | 1 | 97.65 ± 8.19 | 99.28 ± 5.56 | 103.87 ± 6.02 | 8.39 | 5.60 | 5.80 | 6.58 |
| 5 | 103.39 ± 6.65 | 104.66 ± 7.43 | 106.94 ± 10.37 | 6.44 | 7.10 | 9.69 | 7.52 | |
| 10 | 101.25 ± 3.96 | 111.46 ± 7.84 | 108.56 ± 7.72 | 3.91 | 7.04 | 7.11 | 7.22 | |
| Liver | 1 | 91.55 ± 1.26 | 96.89 ± 3.20 | 96.64 ± 2.58 | 1.37 | 3.30 | 2.67 | 3.61 |
| 5 | 98.43 ± 2.51 | 105.49 ± 7.19 | 95.81 ± 5.35 | 2.55 | 6.82 | 5.58 | 6.54 | |
| 10 | 97.52 ± 7.97 | 99.58 ± 9.10 | 102.04 ± 4.22 | 8.17 | 9.14 | 4.14 | 7.13 | |
| Kidney | 1 | 97.32 ± 2.64 | 99.70 ± 4.89 | 98.93 ± 3.29 | 2.72 | 4.90 | 3.32 | 3.53 |
| 5 | 102.10 ± 2.91 | 99.27 ± 3.40 | 99.39 ± 5.51 | 2.85 | 3.42 | 5.54 | 4.02 | |
| 10 | 102.69 ± 8.51 | 105.32 ± 1.51 | 106.34 ± 2.34 | 8.28 | 1.43 | 2.20 | 4.81 | |
| Matrix | Added Concentration (μg/mL or μg/g) | Recovery Rate ( ± S.D., %, n = 5) | Intra-Batch Precision (%, n = 5) | Inter-Batch Precision (%, n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| Urine | 1 | 89.72 ± 7.34 | 86.69 ± 4.21 | 80.56 ± 9.21 | 8.18 | 4.85 | 11.44 | 9.03 |
| 5 | 90.48 ± 6.97 | 85.92 ± 7.94 | 83.17 ± 3.65 | 7.70 | 9.24 | 4.39 | 7.54 | |
| 10 | 91.04 ± 4.14 | 95.70 ± 5.00 | 94.34 ± 5.57 | 4.55 | 5.23 | 5.90 | 5.38 | |
| Liver | 1 | 107.20 ± 2.82 | 109.71 ± 7.67 | 100.72 ± 2.61 | 2.63 | 6.99 | 2.60 | 6.43 |
| 5 | 110.11 ± 4.79 | 110.45 ± 7.99 | 107.07 ± 10.76 | 4.35 | 7.23 | 10.05 | 6.77 | |
| 10 | 103.96 ± 9.75 | 105.83 ± 7.86 | 109.12 ± 8.92 | 9.38 | 7.43 | 8.17 | 8.01 | |
| Kidney | 1 | 110.62 ± 5.50 | 99.35 ± 4.31 | 100.31 ± 3.25 | 4.97 | 4.34 | 3.24 | 6.62 |
| 5 | 109.02 ± 3.72 | 106.40 ± 9.05 | 107.66 ± 9.94 | 3.41 | 8.50 | 9.23 | 7.00 | |
| 10 | 109.17 ± 8.12 | 110.04 ± 6.51 | 109.29 ± 8.16 | 7.44 | 5.92 | 7.47 | 6.47 | |
| Feces | 1 | 81.84 ± 6.70 | 84.54 ± 6.28 | 81.28 ± 7.43 | 8.24 | 7.43 | 9.14 | 7.74 |
| 5 | 87.86 ± 7.59 | 89.57 ± 5.55 | 81.32 ± 5.45 | 8.63 | 6.20 | 6.70 | 7.86 | |
| 10 | 85.14 ± 3.04 | 84.69 ± 3.97 | 88.32 ± 2.12 | 3.56 | 4.69 | 2.40 | 3.83 | |
| Matrix | Added Concentration (μg/mL or μg/g) | Recovery Rate ( ± S.D., %, n = 5) | Intra-Batch Precision (%, n = 5) | Inter-Batch Precision (%, n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| Urine | 1 | 96.11 ± 7.16 | 94.65 ± 10.81 | 101.83 ± 9.66 | 7.45 | 11.42 | 9.49 | 9.58 |
| 5 | 99.61 ± 5.37 | 105.82 ± 8.90 | 103.90 ± 8.66 | 5.39 | 8.42 | 8.34 | 7.34 | |
| 10 | 101.83 ± 5.92 | 110.34 ± 9.18 | 110.92 ± 6.68 | 5.82 | 8.32 | 6.02 | 7.45 | |
| Liver | 1 | 103.52 ± 6.93 | 108.31 ± 9.88 | 97.44 ± 7.51 | 6.70 | 9.12 | 7.70 | 8.62 |
| 5 | 104.94 ± 2.66 | 104.86 ± 9.06 | 94.50 ± 6.44 | 2.53 | 8.64 | 6.82 | 7.83 | |
| 10 | 103.11 ± 7.89 | 99.62 ± 9.34 | 105.79 ± 11.10 | 7.65 | 9.37 | 10.49 | 8.95 | |
| Kidney | 1 | 101.38 ± 7.68 | 92.76 ± 6.99 | 91.59 ± 9.72 | 7.57 | 7.54 | 10.61 | 9.19 |
| 5 | 104.07 ± 6.81 | 91.39 ± 6.11 | 98.31 ± 6.73 | 6.54 | 6.69 | 6.84 | 8.27 | |
| 10 | 102.62 ± 9.81 | 101.35 ± 9.57 | 105.69 ± 9.54 | 9.56 | 9.45 | 9.02 | 8.84 | |
| Feces | 1 | 88.28 ± 8.00 | 86.09 ± 5.68 | 80.07 ± 6.10 | 9.06 | 6.60 | 7.62 | 8.30 |
| 5 | 86.91 ± 8.27 | 84.95 ± 8.53 | 90.40 ± 7.10 | 9.52 | 10.04 | 7.86 | 9.50 | |
| 10 | 82.9 ± 3.42 | 91.16 ± 7.14 | 89.27 ± 3.25 | 4.12 | 7.83 | 3.64 | 5.41 | |
| Matrix | Added Concentration (μg/mL or μg/g) | Recovery Rate ( ± S.D., %, n = 5) | Intra-Batch Precision (%, n = 5) | Inter-Batch Precision (%, n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| Urine | 1 | 96.93 ± 5.54 | 96.11 ± 3.06 | 97.62 ± 5.37 | 5.72 | 3.18 | 5.50 | 4.63 |
| 5 | 98.24 ± 5.90 | 97.13 ± 4.28 | 95.05 ± 8.70 | 6.01 | 4.41 | 9.15 | 6.42 | |
| 10 | 96.39 ± 4.38 | 94.05 ± 3.48 | 94.78 ± 6.05 | 4.54 | 3.70 | 6.38 | 4.75 | |
| Liver | 1 | 78.15 ± 8.94 | 84.63 ± 6.94 | 74.34 ± 6.84 | 11.44 | 8.20 | 9.20 | 10.53 |
| 5 | 79.99 ± 7.11 | 80.70 ± 7.20 | 80.62 ± 6.79 | 8.89 | 8.92 | 8.43 | 8.11 | |
| 10 | 81.25 ± 3.42 | 84.14 ± 5.49 | 74.76 ± 8.97 | 4.21 | 6.52 | 12.00 | 9.22 | |
| Kidney | 1 | 75.34 ± 5.64 | 78.19 ± 5.58 | 82.73 ± 5.39 | 7.48 | 7.13 | 6.51 | 7.64 |
| 5 | 71.59 ± 6.78 | 79.43 ± 8.34 | 80.27 ± 6.65 | 9.48 | 10.50 | 8.29 | 10.22 | |
| 10 | 72.23 ± 5.48 | 75.43 ± 6.62 | 81.62 ± 4.05 | 7.59 | 8.77 | 4.96 | 8.49 | |
| Feces | 1 | 80.41 ± 7.91 | 83.70 ± 5.58 | 80.12 ± 6.78 | 9.83 | 6.66 | 8.46 | 8.09 |
| 5 | 76.98 ± 3.41 | 79.97 ± 4.88 | 82.02 ± 9.63 | 4.43 | 6.10 | 11.74 | 8.06 | |
| 10 | 84.44 ± 4.75 | 82.54 ± 4.76 | 83.15 ± 6.95 | 5.62 | 5.76 | 8.36 | 6.28 | |
| Time (h) | Mean Drug Concentration (μg/g) ± S.D. | ||||
|---|---|---|---|---|---|
| Amoxicillin | Amoxicilloic Acid | Amoxicillin Diketopiperazine | Total Amoxicillin | Sulbactam | |
| 0.5 | 2.503 ± 0.128 | 2.488 ± 0.090 | 0.636 ± 0.029 | 5.627 ± 0.325 | 2.892 ± 0.170 |
| 1 | 3.516 ± 0.223 | 3.037 ± 0.301 | 0.469 ± 0.020 | 7.022 ± 0.491 | 3.499 ± 0.154 |
| 2 | 1.882 ± 0.131 | 2.452 ± 0.213 | 0.298 ± 0.018 | 4.631 ± 0.392 | 2.256 ± 0.189 |
| 4 | 0.630 ± 0.041 | 2.052 ± 0.165 | 0.223 ± 0.044 | 2.905 ± 0.166 | 1.141 ± 0.132 |
| 8 | 0.127 ± 0.017 | 0.692 ± 0.035 | 0.161 ± 0.015 | 0.980 ± 0.039 | 0.346 ± 0.051 |
| 12 | 0.107 ± 0.015 | 0.053 ± 0.016 | 0.112 ± 0.021 | 0.273 ± 0.043 | 0.098 ± 0.040 |
| 24 | ND | ND | ND | ND | 0.038 ± 0.008 |
| 48 | ND | ND | ND | ND | ND |
| Note: Total amoxicillin is the total concentration of amoxicillin, amoxicillinic acid, and amoxicillin diketopiperazine. “ND” means not detected. | |||||
| Time (h) | Mean Drug Concentration (μg/g) ± S.D. | ||||
|---|---|---|---|---|---|
| Amoxicillin | Amoxicilloic Acid | Amoxicillin Diketopiperazine | Total Amoxicillin | Sulbactam | |
| 0.5 | 3.013 ± 0.171 | 2.635 ± 0.149 | 0.620 ± 0.051 | 6.268 ± 0.369 | 3.569 ± 0.193 |
| 1 | 3.432 ± 0.126 | 3.608 ± 0.158 | 0.479 ± 0.035 | 7.520 ± 0.386 | 3.895 ± 0.272 |
| 2 | 1.693 ± 0.117 | 2.908 ± 0.104 | 0.281 ± 0.019 | 4.882 ± 0.343 | 2.093 ± 0.142 |
| 4 | 0.542 ± 0.014 | 1.827 ± 0.164 | 0.205 ± 0.018 | 2.575 ± 0.182 | 0.100 ± 0.068 |
| 8 | 0.110 ± 0.002 | 0.719 ± 0.069 | 0.143 ± 0.016 | 0.972 ± 0.075 | 0.331 ± 0.014 |
| 12 | 0.083 ± 0.018 | 0.044 ± 0.007 | 0.081 ± 0.013 | 0.208 ± 0.015 | 0.080 ± 0.021 |
| 24 | ND | ND | ND | ND | 0.036 ± 0.003 |
| 48 | ND | ND | ND | ND | ND |
| Note: Total amoxicillin is the total concentration of amoxicillin, amoxicillinic acid, and amoxicillin diketopiperazine. “ND” means not detected. | |||||
| Time (h) | Mean Drug Concentration(μg/g) ± S.D. | ||||
|---|---|---|---|---|---|
| Amoxicillin | Amoxicilloic Acid | Amoxicillin Diketopiperazine | Total Amoxicillin | Sulbactam | |
| 0.5 | 0.480 ± 0.013 | 2.115 ± 0.144 | 0.297 ± 0.019 | 2.840 ± 0.175 | 1.646 ± 0.056 |
| 1 | 0.277 ± 0.010 | 2.607 ± 0.257 | 0.249 ± 0.024 | 3.187 ± 0.151 | 1.919 ± 0.150 |
| 2 | 0.234 ± 0.007 | 1.904 ± 0.197 | 0.192 ± 0.016 | 2.436 ± 0.117 | 1.386 ± 0.117 |
| 4 | 0.163 ± 0.002 | 0.726 ± 0.051 | 0.135 ± 0.012 | 1.014 ± 0.047 | 0.963 ± 0.065 |
| 8 | 0.075 ± 0.002 | 0.241 ± 0.018 | 0.107 ± 0.009 | 0.403 ± 0.017 | 0.310 ± 0.018 |
| 12 | ND | 0.064 ± 0.006 | 0.092 ± 0.012 | 0.176 ± 0.023 | 0.068 ± 0.012 |
| 24 | ND | ND | ND | ND | ND |
| Note: Total amoxicillin is the total concentration of amoxicillin, amoxicillinic acid, and amoxicillin diketopiperazine. “ND” means not detected. | |||||
| Time (h) | Mean Drug Concentration (μg/g) ± S.D. | ||||
|---|---|---|---|---|---|
| Amoxicillin | Amoxicilloic Acid | Amoxicillin Diketopiperazine | Total Amoxicillin | Sulbactam | |
| 0.5 | 0.467 ± 0.013 | 2.380 ± 0.117 | 0.309 ± 0.064 | 3.155 ± 0.125 | 1.846 ± 0.102 |
| 1 | 0.251 ± 0.032 | 2.987 ± 0.122 | 0.259 ± 0.012 | 3.497 ± 0.149 | 2.142 ± 0.476 |
| 2 | 0.162 ± 0.003 | 2.026 ± 0.112 | 0.178 ± 0.025 | 2.265 ± 0.153 | 1.457 ± 0.290 |
| 4 | 0.121 ± 0.005 | 0.805 ± 0.063 | 0.135 ± 0.012 | 1.061 ± 0.090 | 0.991 ± 0.135 |
| 8 | 0.069 ± 0.003 | 0.171 ± 0.019 | 0.100 ± 0.011 | 0.340 ± 0.015 | 0.266 ± 0.017 |
| 12 | ND | 0.046 ± 0.002 | 0.099 ± 0.005 | 0.145 ± 0.012 | 0.065 ± 0.005 |
| 24 | ND | ND | ND | ND | ND |
| Note: Total amoxicillin is the total concentration of amoxicillin, amoxicillinic acid, and amoxicillin diketopiperazine. “ND” means not detected. | |||||
| Time (h) | Excretion Rate (%) ± S.D. | ||||
|---|---|---|---|---|---|
| Amoxicillin | Amoxicilloic Acid | Amoxicillin Diketopiperazine | Sulbactam | Total | |
| 0.5 | ND | ND | ND | ND | ND |
| 1 | 0.97 ± 0.08 | 2.52 ± 0.15 | ND | 1.47 ± 0.15 | 4.97 ± 0.35 |
| 2 | 2.11 ± 0.12 | 4.11 ± 0.34 | 1.01 ± 0.09 | 2.73 ± 0.13 | 9.95 ± 0.75 |
| 4 | 4.13 ± 0.21 | 6.88 ± 0.50 | 1.72 ± 0.12 | 5.63 ± 0.36 | 18.36 ± 0.81 |
| 8 | 6.36 ± 0.46 | 12.59 ± 1.01 | 3.08 ± 0.23 | 12.31 ± 0.81 | 34.36 ± 1.25 |
| 12 | 8.42 ± 0.34 | 16.68 ± 1.28 | 4.69 ± 0.35 | 15.01 ± 1.05 | 44.78 ± 1.66 |
| 24 | 10.12 ± 0.62 | 20.25 ± 1.10 | 5.50 ± 0.40 | 16.44 ± 1.03 | 52.30 ± 1.84 |
| 48 | 10.83 ± 0.72 | 24.36 ± 1.41 | 6.99 ± 0.50 | 17.44 ± 1.42 | 59.62 ± 2.50 |
| 72 | 10.98 ± 0.65 | 24.26 ± 1.36 | 7.15 ± 0.42 | 18.22 ± 1.30 | 60.61 ± 2.13 |
| Note: Total is the total excretion rate of amoxicillin, amoxicillinic acid, amoxicillin diketopiperazine, and sulbactam. “ND” means not detected. | |||||
| Time (h) | Excretion Rate (%) ± S.D. | ||||
|---|---|---|---|---|---|
| Amoxicillin | Amoxicilloic Acid | Amoxicillin Diketopiperazine | Sulbactam | Total | |
| 0.5 | ND | ND | ND | ND | ND |
| 1 | 1.20 ± 0.06 | 2.55 ± 0.13 | ND | 1.54 ± 0.18 | 5.28 ± 0.30 |
| 2 | 2.85 ± 0.17 | 3.74 ± 0.12 | 0.97 ± 0.03 | 2.84 ± 0.11 | 10.41 ± 0.76 |
| 4 | 5.09 ± 0.46 | 6.60 ± 0.44 | 1.80 ± 0.11 | 6.02 ± 0.32 | 19.50 ± 0.73 |
| 8 | 7.01 ± 0.41 | 12.80 ± 0.72 | 3.23 ± 0.24 | 10.63 ± 0.76 | 33.67 ± 1.25 |
| 12 | 9.35 ± 0.53 | 17.14 ± 0.68 | 4.35 ± 0.19 | 14.97 ± 1.19 | 45.81 ± 1.46 |
| 24 | 11.11 ± 0.87 | 21.87 ± 1.12 | 5.83 ± 0.33 | 16.81 ± 1.13 | 55.63 ± 1.14 |
| 48 | 11.87 ± 0.62 | 22.52 ± 1.21 | 8.05 ± 0.47 | 18.11 ± 1.04 | 60.55 ± 1.55 |
| 72 | 11.68 ± 0.76 | 23.52 ± 1.04 | 8.40 ± 0.62 | 19.02 ± 1.38 | 62.62 ± 1.73 |
| Note: Total is the total excretion rate of amoxicillin, amoxicillinic acid, amoxicillin diketopiperazine, and sulbactam. “ND” means not detected. | |||||
| Time (h) | Excretion Rate (%) ± S.D. | |||
|---|---|---|---|---|
| Amoxicilloic Acid | Amoxicillin Diketopiperazine | Sulbactam | Total | |
| 0.5 | ND | ND | ND | ND |
| 1 | ND | ND | ND | ND |
| 2 | 0.01 | 0.01 | 0.02 | 0.04 |
| 4 | 0.02 | 0.01 | 0.02 | 0.05 |
| 8 | 0.02 | 0.02 | 0.02 | 0.06 |
| 12 | 0.12 ± 0.01 | 0.09 | 0.23 ± 0.04 | 0.44 ± 0.02 |
| 24 | 0.95 ± 0.07 | 1.36 ± 0.08 | 2.29 ± 0.13 | 3.61 ± 0.11 |
| 48 | 2.62 ± 0.11 | 1.85 ± 0.15 | 4.39 ± 0.14 | 8.85 ± 0.12 |
| 72 | 2.85 ± 0.12 | 2.14 ± 0.16 | 4.35 ± 0.11 | 9.54 ± 0.26 |
| Note: Total is the total excretion rate of amoxicillin, amoxicillinic acid, amoxicillin diketopiperazine, and sulbactam. “ND” means not detected. | ||||
| Time (h) | Excretion rate (%) ± S.D. | |||
|---|---|---|---|---|
| Amoxicilloic Acid | Amoxicillin Diketopiperazine | Sulbactam | Total | |
| 0.5 | ND | ND | ND | ND |
| 1 | ND | ND | ND | ND |
| 2 | 0.03 | 0.01 | 0.03 | 0.08 |
| 4 | 0.04 | 0.02 | 0.06 | 0.12 |
| 8 | 0.08 | 0.05 | 0.17 ± 0.01 | 0.29 |
| 12 | 1.14 ± 0.08 | 1.15 ± 0.07 | 1.46 ± 0.07 | 3.76 ± 0.16 |
| 24 | 2.12 ± 0.12 | 1.46 ± 0.08 | 3.88 ± 0.11 | 7.46 ± 0.11 |
| 48 | 3.08 ± 0.11 | 2.50 ± 0.14 | 5.09 ± 0.14 | 10.67 ± 0.25 |
| 72 | 2.87 ± 0.17 | 2.42 ± 0.11 | 5.32 ± 0.15 | 10.60 ± 0.24 |
| Note: Total is the total excretion rate of amoxicillin, amoxicillinic acid, amoxicillin diketopiperazine, and sulbactam. “ND” means not detected. | ||||
References
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in china. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The use of antibiotics and antimicrobial resistance in veterinary medicine, a complex phenomenon: A narrative review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sanchez-Melsio, A.; Borrego, C.M.; Barcelo, D.; Balcazar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F. The resistance tsunami, antimicrobial stewardship, and the golden age of microbiology. Vet. Microbiol. 2014, 171, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Berdigaliyev, N.; Aljofan, M. An overview of drug discovery and development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef]
- Brodniewicz, T.; Grynkiewicz, G. Preclinical drug development. Acta Pol. Pharm. 2010, 67, 578–585. [Google Scholar] [PubMed]
- Bremner, J.B.; Ambrus, J.I.; Samosorn, S. Dual action-based approaches to antibacterial agents. Curr. Med. Chem. 2007, 14, 1459–1477. [Google Scholar] [CrossRef]
- Pokrovskaya, V.; Baasov, T. Dual-acting hybrid antibiotics: A promising strategy to combat bacterial resistance. Expert Opin. Drug Discov. 2010, 5, 883–902. [Google Scholar] [CrossRef]
- Beskid, G.; Siebelist, J.; Mcgarry, C.M.; Cleeland, R.; Chan, K.; Keith, D.D. In vivo evaluation of a dual-action antibacterial, ro 23-9424, compared to cefotaxime and fleroxacin. Chemotherapy 1990, 36, 109–116. [Google Scholar] [CrossRef]
- Georgopapadakou, N.H.; Bertasso, A.; Chan, K.K.; Chapman, J.S.; Cleeland, R.; Cummings, L.M.; Dix, B.A.; Keith, D.D. Mode of action of the dual-action cephalosporin ro 23-9424. Antimicrob. Agents Chemother. 1989, 33, 1067–1071. [Google Scholar] [CrossRef]
- Cristancho, O.C.; de Freitas, S.M.; Pruccoli, L.; Fonseca, N.N.; de Azevedo, L.L.; Kummerle, A.E.; Guedes, I.A.; Dardenne, L.E.; Leomil, C.L.; Guimaraes, M.J.; et al. Design, synthesis, and biological evaluation of new thalidomide-donepezil hybrids as neuroprotective agents targeting cholinesterases and neuroinflammation. RSC Med. Chem. 2022, 13, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Multi-targeting by monotherapeutic antibacterials. Nat. Rev. Drug Discov. 2007, 6, 41–55. [Google Scholar] [CrossRef]
- Christenson, J.G.; Chan, K.K.; Cleeland, R.; Dix-Holzknecht, B.; Farrish, H.J.; Patel, I.H.; Specian, A. Pharmacokinetics of ro 23-9424, a dual-action cephalosporin, in animals. Antimicrob. Agents Chemother. 1990, 34, 1895–1900. [Google Scholar] [CrossRef]
- Okimoto, N.; Kurihara, T.; Honda, N.; Asaoka, N.; Fujita, K.; Ohba, H.; Nakamura, J. Clinical effect of ampicillin with beta-lactamase inhibitor (sulbactam/ampicillin) on community-acquired pneumonia in the elderly. J. Infect. Chemother. 2003, 9, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V.; Nguyen, H.T.; Ho, D.H.; Leblanc, B.; Bodey, G.P. In vitro activity of ro 23-9424, a dual-action antibacterial agent, against bacterial isolates from cancer patients compared with those of other agents. Antimicrob. Agents Chemother. 1992, 36, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.; Murray, F.E.; Cole, A.T.; Turnbull, G.M.; Lettis, S.; Hawkey, C.J. Ranitidine bismuth citrate and aspirin-induced gastric mucosal injury. Aliment. Pharmacol. Ther. 1993, 7, 515–521. [Google Scholar] [CrossRef]
- Sutherland, R.; Croydon, E.A.P.; Rolinson, G.N. Amoxycillin: A new semi-synthetic penicillin. Br. Med. J. 1972, 3, 13–16. [Google Scholar] [CrossRef]
- De Marco, B.A.; Natori, J.S.H.; Fanelli, S.; Tótoli, E.G.; Salgado, H.R.N. Characteristics, properties and analytical methods of amoxicillin: A review with green approach. Crit. Rev. Anal. Chem. 2017, 47, 267–277. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Moller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Acuna, C.; Rabasseda, X. Amoxicillin-sulbactam: A clinical and therapeutic review. Drugs Today 2001, 37, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Soutric, J.; Bantar, C.; Caruso, N.; Heguilen, R.; Casellas, J.J.; Casellas, J.M.; Farinati, A.; Jasovich, A.; Arenoso, H.; Rodriguez, M. Review of pharmacokinetic, pharmacodynamic and clinical studies with a modern combination of amoxicillin/sulbactam. Chemotherapy 2006, 52, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Akova, M. Sulbactam-containing β-lactamase inhibitor combinations. Clin. Microbiol. Infect. 2008, 14, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Bantar, C.; Fernandez, C.L.; Berger, M.A.; Soutric, J.L.; Arenoso, H.J. Intravenous amoxicillin-sulbactam against escherichia coli: Optimizing the dose, component ratio and infusion time using a human pharmacodynamic model. J. Chemother. 2009, 21, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Yang, G.P.; Li, Z.J.; Peng, X.D.; Fan, J.H.; Liu, Z.Q. Simultaneous analysis of amoxicillin and sulbactam in human plasma by hplc-dad for assessment of bioequivalence. J. Chromatogr. B 2011, 879, 2000–2004. [Google Scholar] [CrossRef]
- Jasys, V.J. Process for the Preparation of Penicillanic Acid Esters. DE. Invention Patent DE3270720, 28 May 1986. [Google Scholar]
- Bigham, E.C. Bis-Esters of Methanediol with Penicillins and Penicillanic acid 1,1-Dioxide. U.S. Invention Patent US4377524, 22 March 1983. [Google Scholar]
- Zhao, F.K.; Shi, R.B.; Sun, Y.B.; Yang, S.Y.; Chen, L.Z.; Fang, B.H. A comprehensive study to identify major metabolites of an amoxicillin-sulbactam hybrid molecule in rats and its metabolic pathway using uplc-q-tof-ms/ms. Metabolites 2022, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N. Simultaneous determination of amoxicillin and chloramphenicol and their drug interaction study by the validated uplc method. J. Taibah Univ. Sci. 2016, 10, 755–765. [Google Scholar] [CrossRef]
- Nidal, B.; Shorouq, W.; Fuad, A.R. A validated stability-indicating hplc method for simultaneous determination of amoxicillin and enrofloxacin combination in an injectable suspension. Pharm 2017, 85, 6. [Google Scholar]
- Dou, A.M.; Hosmanová, R. Rapid determination of amoxicillin in premixes by hplc. J. Pharm. Biomed. Anal. 2005, 37, 373–377. [Google Scholar]
- Ibrahim, F.A.; Nasr, J.J.M. Direct determination of ampicillin and amoxicillin residues in food samples after aqueous sds extraction by micellar liquid chromatography with uv detection. Anal. Methods 2014, 6, 1523. [Google Scholar] [CrossRef]
- Shah, A.J.; Adlard, M.W.; Stride, J.D. A sensitive assay for clavulanic acid and sulbactam in biological fluids by high-performance liquid chromatography and precolumn derivatization. J. Pharm. Biomed. Anal. 1990, 8, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Gamba, V.; Dusi, G. Liquid chromatography with fluorescence detection of amoxicillin and ampicillin in feeds using pre-column derivatization. Anal. Chim. Acta 2003, 483, 69–72. [Google Scholar] [CrossRef]
- Kaizhou, X.; Longfei, J.; Dong, X.; Huisheng, G.; Xing, X.; Yuping, H.; Xuesen, C.; Wenbin, B.; Guojun, D.; Jinyu, W. Simultaneous determination of amoxicillin and ampicillin in eggs by reversed-phase high-performance liquid chromatography with fluorescence detection using pre-column derivatization. J. Chromatogr. Sci. 2012, 50, 620–624. [Google Scholar]
- Colin, P.; De Bock, L.; T’jollyn, H.; Boussery, K.; Van Bocxlaer, J. Development and validation of a fast and uniform approach to quantify β-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography–electrospray-tandem mass spectrometry. Talanta 2013, 103, 285–293. [Google Scholar] [CrossRef] [PubMed]
- De Baere, S.; Cherlet, M.; Baert, K.; De Backer, P. Quantitative analysis of amoxycillin and its major metabolites in animal tissues by liquid chromatography combined with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002, 74, 1393. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jia, L.; Xie, X.; Xie, K.; Wang, J.; Liu, J.; Cui, L.; Zhang, G.; Dai, G.; Wang, J. Quantitative analysis of amoxicillin, its major metabolites and ampicillin in eggs by liquid chromatography combined with electrospray ionization tandem mass spectrometry. Food Chem. 2016, 192, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, B.; Diao, Z.; Zhao, M.; Xie, K.; Zhang, P.; Wang, X.; Zhang, T.; Wang, J. Development and validation of an hplc-esi/ms/ms method for the determination of amoxicillin, its major metabolites, and ampicillin residues in chicken tissues. Molecules 2019, 24, 2652. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Leng, B.; Jiang, J.; Zhang, L.; Guo, N.; Shen, C. An hplc-ms/ms method for determination of sulbactam in human plasma and its pharmacokinetic application in critically ill patients with augmented renal clearance. Int. J. Clin. Pharmacol. Ther. 2023, 61, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pang, M.; Xie, X.; Zhao, M.; Xie, K.; Zhang, Y.; Zhao, X.; Wang, Y.J.; Wang, R.; Wu, H. Quantitative analysis of amoxicillin, amoxicillin major metabolites, and ampicillin in chicken tissues via ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Food Anal. Meth. 2017, 10, 3292–3305. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Jiang, Y.; Du, Z. Rapid and simultaneous determination of amoxicillin, penicillin g, and their major metabolites in bovine milk by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 533–540. [Google Scholar] [CrossRef]
- Reyns, T.; Cherlet, M.; De Baere, S.; De Backer, P.; Croubels, S. Rapid method for the quantification of amoxicillin and its major metabolites in pig tissues by liquid chromatography-tandem mass spectrometry with emphasis on stability issues. J. Chromatogr. B 2008, 861, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Wang, J.R.; Lu, Y.L. Pharmacokinetics of sulbactam and ampicillin in mice and in dogs. Yao Xue Xue Bao = Acta Pharm. Sin. 1990, 25, 406–411. [Google Scholar]
- Kukanich, K.; Woodruff, K.; Bieberly, Z.; Papich, M.G.; Kukanich, B. Evaluation of urine concentrations of amoxicillin and clavulanate in cats. J. Vet. Intern. Med. 2021, 35, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Foulds, G. Pharmacokinetics of sulbactam/ampicillin in humans: A review. Rev. Infect. Dis. 1986, 8 (Suppl. S5), S503–S511. [Google Scholar] [CrossRef] [PubMed]
- Krasucka, D.; Kowalski, C.J. Pharmacokinetic parameters of amoxicillin in pigs and poultry. Acta Pol. Pharm. 2010, 67, 729–732. [Google Scholar] [PubMed]
- Van Vooren, S.; De Waele, J.J.; Boelens, J.; Polet, M.; Stove, V.; Vanhaecke, L.; Verstraete, A.G. Development and validation of a liquid chromatography high-resolution mass spectrometry orbitrap method for the sensitive quantification of amoxicillin, piperacillin, tazobactam and meropenem in human faeces. Anal. Chim. Acta 2021, 1177, 338760. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, J.; Xiong, W.; Zeng, Z. Rapid determination of amoxicillin in porcine tissues by uplc-ms/ms with internal standard. J. Food Compos. Anal. 2020, 92, 103578. [Google Scholar] [CrossRef]
- Bin, W.U.; Jing, L.I.; Amp, M.F. Determination of the residue of amoxicillin in animal origin food stuffs by lc-ms/ms. J. Anhui Agric. Sci. 2015, 43, 280–283. [Google Scholar]
- Xian-Cheng, Y.E.; De-Qiu, Z.; Guo-Rong, F. Determination of sulbactam sodium concentration in human urine by lc-ms/ms and its kinetics study. China Pharm. 2012, 23, 531–533. [Google Scholar]
- Elsheikh, H.A.; Taha, A.A.; Khalafalla, A.E.; Osman, I.A.; Wasfi, I.A. Pharmacokinetics of amoxicillin trihydrate in desert sheep and nubian goats. Vet. Res. Commun. 1999, 23, 507–514. [Google Scholar] [CrossRef]
- Campoli-Richards, D.M.; Brogden, R.N. Sulbactam/ampicillin. A review of its antibacterial activity, pharmacokinetic properties, and therapeutic use. Drugs 1987, 33, 577–609. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, X.; Zhao, M.; Guo, B.; Cao, G.; Yu, J.; Chen, Y.; Zhang, J. Rapid and simultaneous quantitation of amoxicillin and clavulanic acid in human plasma and urine by ultra-performance liquid chromatography tandem mass spectrometry and its application to a pharmacokinetic study. Anal. Sci. 2016, 32, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Foulds, G.; Stankewich, J.P.; Marshall, D.C.; O’Brien, M.M.; Hayes, S.L.; Weidler, D.J.; Mcmahon, F.G. Pharmacokinetics of sulbactam in humans. Antimicrob. Agents Chemother. 1983, 23, 692–699. [Google Scholar] [CrossRef] [PubMed]










| Parameter | Analyte | Matrix | |||
|---|---|---|---|---|---|
| Liver | Kidneys | Urine | Feces | ||
| LOD (μg/mL or μg/g) | AMO | 0.01 | 0.01 | 0.01 | — |
| AMA | 0.01 | 0.01 | 0.01 | 0.025 | |
| DIKETO | 0.0025 | 0.0025 | 0.0025 | 0.01 | |
| SBT | 0.01 | 0.01 | 0.01 | 0.025 | |
| LOQ (μg/mL or μg/g) | AMO | 0.025 | 0.025 | 0.025 | — |
| AMA | 0.025 | 0.025 | 0.025 | 0.05 | |
| DIKETO | 0.01 | 0.01 | 0.01 | 0.025 | |
| SBT | 0.025 | 0.025 | 0.025 | 0.05 | |
| Matrix | Metabolite | Group | Equation | Elimination Half-Life (h) |
|---|---|---|---|---|
| Liver | Total amoxicillin and its metabolites | AS group | C = 8.71e−0.28t | 2.44 ± 0.05 * |
| Mixture group | C = 9.35e−0.31t | 2.32 ± 0.05 | ||
| Amoxicillin | AS group | C = 2.33e−0.29t | 2.40 ± 0.06 | |
| Mixture group | C = 2.16e−0.30t | 2.30 ± 0.11 | ||
| Amoxicillinic acid | AS group | C = 7.84e−0.38t | 1.82 ± 0.20 * | |
| Mixture group | C = 9.03e−0.41t | 1.70 ± 0.11 | ||
| Sulbactam | AS group | C = 3.93e−0.31t | 2.21 ± 0.30 | |
| Mixture group | C = 3.45e−0.31t | 2.17 ± 0.20 | ||
| Kidney | Total amoxicillin and its metabolites | AS group | C = 3.36e−0.26t | 2.74 ± 0.10 * |
| Mixture group | C = 3.56e−0.28t | 2.54 ± 0.04 | ||
| Amoxicillinic acid | AS group | C = 3.22e−0.33t | 2.11 ± 0.08 ** | |
| Mixture group | C = 3.89e−0.38t | 1.84 ± 0.10 | ||
| Sulbactam | AS group | C = 2.95e−0.30t | 2.28 ± 0.15 | |
| Mixture group | C = 3.12e−0.32t | 2.18 ± 0.08 |
| Time (min) | Flow Rate (μL/min) | 0.1% Formic Acid in Acetonitrile (%) | 0.1% Formic Acid in Water (%) |
|---|---|---|---|
| 0.0 | 350 | 10 | 90 |
| 0.5 | 350 | 10 | 85 |
| 1.0 | 350 | 90 | 10 |
| 3.5 | 350 | 90 | 10 |
| 3.8 | 350 | 10 | 90 |
| 5.0 | 350 | 10 | 90 |
| Analyte | Precursor Ions (m/z) | Product Ions (m/z) | Declustering Potential (V) | Collision Energy (eV) |
|---|---|---|---|---|
| AMO | 366.0 | 114.1 * | 49 | 27 |
| 208.1 | 54 | 19 | ||
| AMA | 383.8 | 323.2 * | 38 | 18 |
| 367.1 | 32 | 15 | ||
| DIKETO | 365.7 | 160.3 * | 53 | 27 |
| 207.3 | 48 | 19 | ||
| AMP | 349.7 | 106.1 * | 26 | 17 |
| 192.1 | 30 | 18 |
| Time (min) | Flow Rate (μL/min) | 0.1% Formic Acid in Acetonitrile (%) | 0.1% Formic Acid in Water (%) |
|---|---|---|---|
| 0.0 | 300 | 5 | 95 |
| 1.0 | 300 | 30 | 70 |
| 2.0 | 300 | 90 | 10 |
| 4.0 | 300 | 90 | 10 |
| 4.2 | 300 | 5 | 95 |
| 6.0 | 300 | 5 | 95 |
| Analyte | Precursor Ions (m/z) | Product Ions (m/z) | Declustering Potential (V) | Collision Energy (eV) |
|---|---|---|---|---|
| Sulbactam | 232.2 | 140.0 * | −35 | −17 |
| 96 | −35 | −17 | ||
| 64 | −35 | −48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Sun, X.; Li, J.; Du, J.; Wu, Z.; Liu, S.; Chen, L.; Fang, B. A Comprehensive Study to Determine the Residual Elimination Pattern of Major Metabolites of Amoxicillin–Sulbactam Hybrid Molecules in Rats by UPLC–MS/MS. Molecules 2024, 29, 2169. https://doi.org/10.3390/molecules29102169
Zhao F, Sun X, Li J, Du J, Wu Z, Liu S, Chen L, Fang B. A Comprehensive Study to Determine the Residual Elimination Pattern of Major Metabolites of Amoxicillin–Sulbactam Hybrid Molecules in Rats by UPLC–MS/MS. Molecules. 2024; 29(10):2169. https://doi.org/10.3390/molecules29102169
Chicago/Turabian StyleZhao, Feike, Xueyan Sun, Jian Li, Junyuan Du, Zhiyi Wu, Shujuan Liu, Liangzhu Chen, and Binghu Fang. 2024. "A Comprehensive Study to Determine the Residual Elimination Pattern of Major Metabolites of Amoxicillin–Sulbactam Hybrid Molecules in Rats by UPLC–MS/MS" Molecules 29, no. 10: 2169. https://doi.org/10.3390/molecules29102169




