High-Efficiency Ag-Modified ZnO/g-C3N4 Photocatalyst with 1D-0D-2D Morphology for Methylene Blue Degradation
Abstract
:1. Introduction
2. Results and Discussion
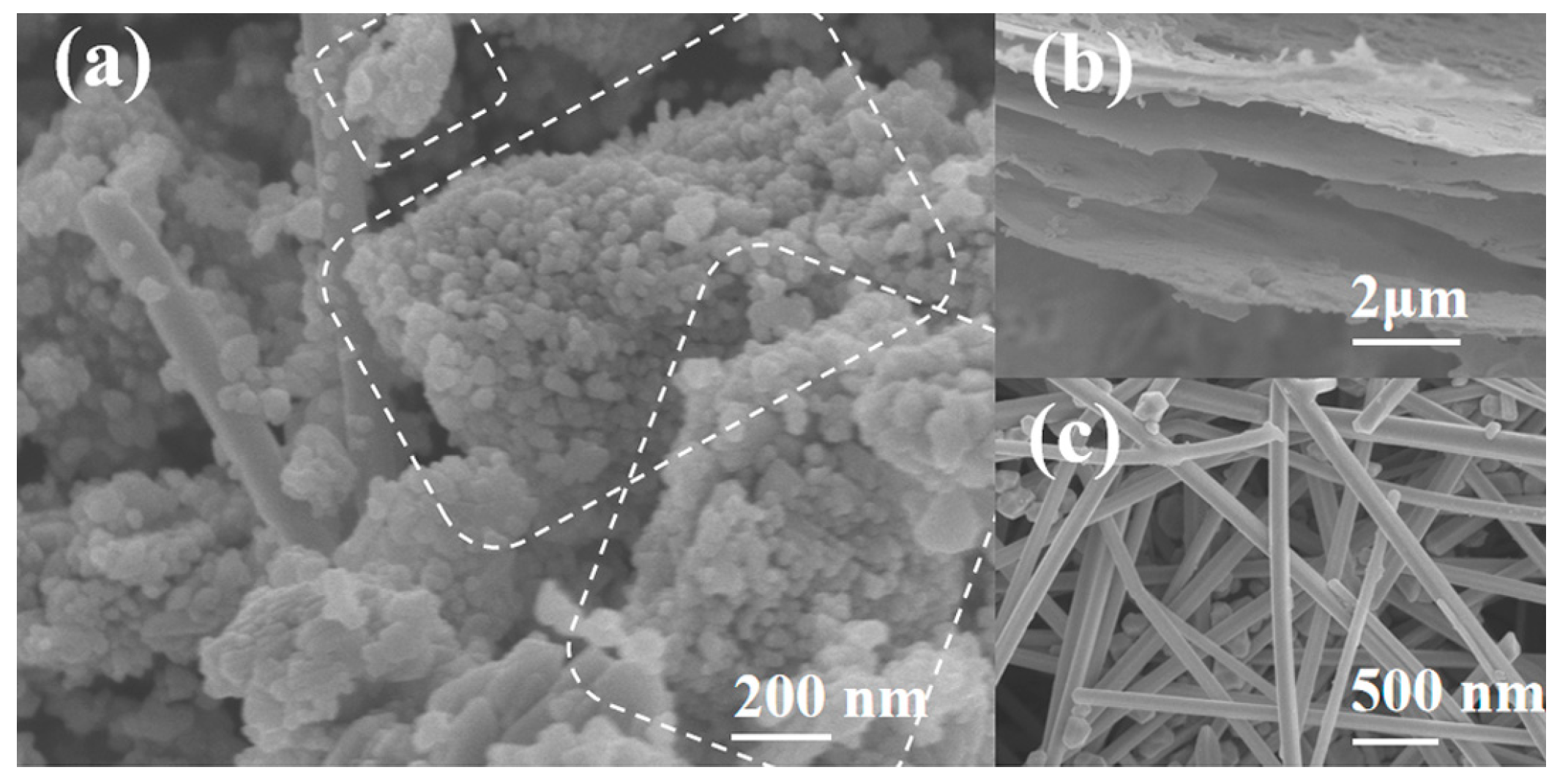
2.1. Structural, Morphological, and Elemental Characterization
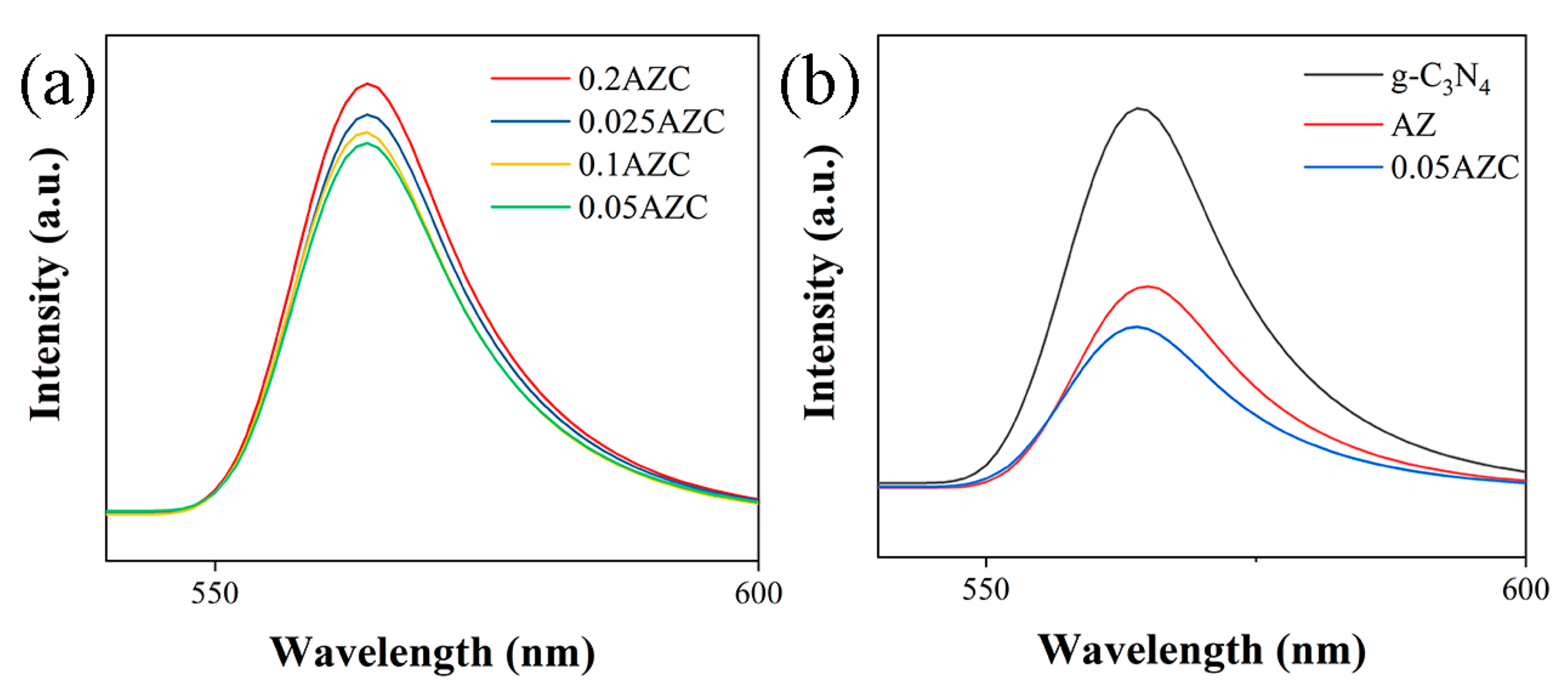
2.2. Optical and Electrochemical Properties
2.3. Photocatalytic Properties
2.4. Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of 0D ZnO Nanoparticles
3.3. Synthesis of 1D Ag Nanowires
3.4. Synthesis of 2D g-C3N4 Nanosheets
3.5. Synthesis of Photocatalyst
3.6. Characterizations
3.7. Photocatalytic Degradation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alguacil, F.J.; López, F.A. Organic Dyes versus Adsorption Processing. Molecules 2021, 26, 5440. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Z.; Fatima, R.; Kim, J.-O. Experimental studies on water matrix and influence of textile effluents on photocatalytic degradation of organic wastewater using Fe–TiO2 nanotubes: Towards commercial application. Environ. Res. 2021, 197, 111120. [Google Scholar] [CrossRef]
- Choi, M.; Singh, N.; Son, S.; Kim, J.H.; Kang, M.; Park, S.H.; Choi, D.H.; Hong, C.S.; Kim, J.S. A post-synthetically modified porous organic polymer for photocatalytic water purification. Mater. Chem. Front. 2023, 7, 2085–2092. [Google Scholar] [CrossRef]
- Garazhian, Z.; Rezaeifard, A.; Jafarpour, M.; Farrokhi, A. {Mo72Fe30} Nanoclusters for the Visible-Light-Driven Photocatalytic Degradation of Organic Dyes. ACS Appl. Nano Mater. 2020, 3, 648–657. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Li, C.; Xu, Z.; Jiang, B. Emulsifier-modulated covalent organic frameworks for photocatalytic degradation of organic dyes. New J. Chem. 2023, 47, 2659–2665. [Google Scholar] [CrossRef]
- Zhou, Y.; Elchalakani, M.; Liu, H.; Briseghella, B.; Sun, C. Photocatalytic concrete for degrading organic dyes in water. Environ. Sci. Pollut. Res. 2022, 29, 39027–39040. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Cao, X.; Dong, J.; Ning, J.; Zhang, F.; Wang, X.; Cheng, Y.; Kou, H.; Zhang, W. Study on the Photocatalytic Properties of Flower-Shaped SnO2. Nanomaterials 2022, 12, 3419. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kweon, D.H.; Jang, B.J.; Ju, M.J.; Baek, J. Enhancing the Photocatalytic Activity of TiO2 Catalysts. Adv. Sustain. Syst. 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, B.; Lin, S. p-type ZnO for photocatalytic water splitting. APL Mater. 2022, 10, 030901. [Google Scholar] [CrossRef]
- Kumaran, N.N.; Muraleedharan, K. Photocatalytic activity of ZnO and Sr2+ doped ZnO nanoparticles. J. Water Process Eng. 2017, 17, 264–270. [Google Scholar] [CrossRef]
- Suresh, S.; Subash, B.; Karthikeyan, S. Electrical, optical and photocatalytic properties of Ti-loaded ZnO/ZnO and Ti-loaded ZnO nanospheres. J. Iran. Chem. Society 2017, 14, 1591–1600. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, E.; Nuñez-Briones, A.G.; García-Cerda, L.A.; Peralta-Rodríguez, R.D.; Montes-Luna, A.J. One-step synthesis of ZnO and Ag/ZnO heterostructures and their photocatalytic activity. Ceram. Int. 2018, 44, 6176–6180. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Cai, S.; Zhang, W.; Zhang, X.; Wu, T.; Wang, C.; Foss, M.; Yang, R. Plasmon-enhanced photocatalytic properties of Au/ZnO nanowires. Appl. Surf. Sci. 2022, 583, 152539. [Google Scholar] [CrossRef]
- Arifin, M.; Roza, L.; Fauzia, V. Bayberry-like Pt nanoparticle decorated ZnO nanorods for the photocatalytic application. Results Phys. 2019, 15, 102678. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Koutavarapu, R.; Lee, S.H.; Arumugam, M.; Kim, J.H.; Choi, M.Y. ZnO supported Au/Pd bimetallic nanocomposites for plasmon improved photocatalytic activity for methylene blue degradation under visible light irradiation. Appl. Surf. Sci. 2019, 496, 143665. [Google Scholar] [CrossRef]
- Peng, J.; Lu, T.; Ming, H.; Ding, Z.; Yu, Z.; Zhang, J.; Hou, Y. Enhanced Photocatalytic Ozonation of Phenol by Ag/ZnO Nanocomposites. Catalysts 2019, 9, 1006. [Google Scholar] [CrossRef]
- Sarma, B.; Sarma, B.K. Fabrication of Ag/ZnO heterostructure and the role of surface coverage of ZnO microrods by Ag nanoparticles on the photophysical and photocatalytic properties of the metal-semiconductor system. Appl. Surf. Sci. 2017, 410, 557–565. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Yin, Q.; Wei, C.; Lin, S.; Hoffman, T.B.; Zhao, Y.; Edgar, J.H.; Chen, Q.; Lau, S.P.; et al. Distinctive in-Plane Cleavage Behaviors of Two-Dimensional Layered Materials. ACS Nano 2016, 10, 8980–8988. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; An, W.; Gu, F.; Cui, L.; He, X.; Fan, M. 2D layered materials: Structures, synthesis, and electrocatalytic applications. Green Chem. 2023, 25, 6149–6169. [Google Scholar] [CrossRef]
- Rajesh, D.; Francis, M.K.; Bhargav, P.B.; Nafis, A.; Balaji, C. 2D layered nickel-cobalt double hydroxide nano sheets @ 1D silver nanowire-graphitic carbon nitrides for high performance super capacitors. J. Alloys Compd. 2022, 898, 162803. [Google Scholar] [CrossRef]
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Moshfegh, A.Z.; Ramakrishna, S. Graphitic carbon nitride (g-C3N4)-based photocatalysts for solar hydrogen generation: Recent advances and future development directions. J. Mater. Chem. A 2017, 5, 23406–23433. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Li, M.; Wang, Q.; Gao, J.; Li, K.; Lei, L. Graphitic carbon nitride (g-C3N4) as a sustainable heterogeneous photocatalyst for metal free and oxygen-tolerant photo-atom transfer radical polymerization (photo-ATRP). Green Chem. 2021, 23, 9617–9624. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Li, H.; Yin, S.; Sato, T.; Wang, Y. Enhanced Photocatalytic Performance of Luminescent g-C3N4 Photocatalyst in Darkroom. Nanoscale Res. Lett. 2016, 11, 91. [Google Scholar] [CrossRef]
- Sano, T.; Koike, K.; Hori, T.; Hirakawa, T.; Ohko, Y.; Takeuchi, K. Removal of methyl mercaptan with highly-mobile silver on graphitic carbon-nitride (g-C3N4) photocatalyst. Appl. Catal. B Environ. 2016, 198, 133–141. [Google Scholar] [CrossRef]
- Jiang, X.; Qiao, K.; Feng, Y.; Sun, L.; Jiang, N.; Wang, J. Self-assembled synthesis of porous sulfur-doped g-C3N4 nanotubes with efficient photocatalytic degradation activity for tetracycline. J. Photochem. Photobiol. A Chem. 2022, 433, 114194. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Chen, M.; Wang, J.; Wang, L. In situ growth strategy synthesis of single-atom nickel/sulfur co-doped g-C3N4 for efficient photocatalytic tetracycline degradation and CO2 reduction. Chem. Eng. J. 2022, 442, 136208. [Google Scholar] [CrossRef]
- Li, W.; Zhou, L.; Xie, L.; Kang, K.; Xu, J.; Chai, X. N-Fe-Gd co-doped TiO2/g-C3N4 nanosheet hybrid composites with superior photocatalytic dye degradation. Adv. Compos. Hybrid Mater. 2022, 5, 481–490. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, X.; Wu, Y.; Su, Y.; Tang, H. 3D/2D direct Z-scheme heterojunctions of hierarchical TiO2 microflowers/g-C3N4 nanosheets with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 2019, 149, 618–626. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Peng, L.; Zhang, Q.; She, X.; Tang, H.; Liu, Q. Dual transfer channels of photo-carriers in 2D/2D/2D sandwich-like ZnIn2S4/g-C3N4/Ti3C2 MXene S-scheme/Schottky heterojunction for boosting photocatalytic H2 evolution. Chin. J. Catal. 2022, 43, 2720–2731. [Google Scholar] [CrossRef]
- Bayan, S.; Gogurla, N.; Ghorai, A.; Ray, S.K. Förster Resonance Energy Transfer Mediated Charge Separation in Plasmonic 2D/1D Hybrid Heterojunctions of Ag–C3N4/ZnO for Enhanced Photodetection. ACS Appl. Nano Mater. 2019, 2, 3848–3856. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Ma, J.; Liu, Z.; Ma, L.; Lei, S.; Fang, J.; Long, C. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 2018, 341, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Faraji, M.; Eghbali, P. Facile fabrication of mpg-C3N4/Ag/ZnO nanowires/Zn photocatalyst plates for photodegradation of dye pollutant. J. Photochem. Photobiol. A Chem. 2020, 400, 112665. [Google Scholar] [CrossRef]
- Orcutt, E.K.; Varapragasam, S.J.; Peterson, Z.C.; Andriolo, J.M.; Skinner, J.L.; Grumstrup, E.M. Ultrafast Charge Injection in Silver-Modified Graphitic Carbon Nitride. ACS Appl. Mater. Interfaces 2023, 15, 15478–15485. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, S.; Huang, R.; Zhang, Q.; Le, T.-T.; Cheng, E.; Yue, R.; Hu, Z.; Chen, Z. Construction of Ni-doped SnO2-SnS2 heterojunctions with synergistic effect for enhanced photodegradation activity. J. Hazard. Mater. 2019, 368, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Sharma, R.; Nehra, S.P.; Sharma, A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.R.; Yang, M.; Choi, S.-I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.-Y.; Camargo, P.H.C.; Ribeiro, S.J.L.; Xia, Y. Facile Synthesis of Sub-20 nm Silver Nanowires through a Bromide-Mediated Polyol Method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fischer, J.M.T.A.; Li, Q.; Hu, J.; Tang, Q.; Wang, H.; Wu, Z.; Hankel, M.; Searles, D.J.; Wang, L. Enhanced CO2 photocatalytic reduction on alkali-decorated graphitic carbon nitride. Appl. Catal. B Environ. 2017, 216, 146–155. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-nanostructure for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, J.; Li, G.; Xu, C. Enhanced photocatalytic activity of P-type (K, Fe) co-doped g-C3N4 synthesized in self-generated NH3 atmosphere. Appl. Surf. Sci. 2019, 470, 99–106. [Google Scholar] [CrossRef]
- Petit, C.; Seredych, M.; Bandosz, T.J. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. J. Mater. Chem. 2009, 19, 9176–9185. [Google Scholar] [CrossRef]
- Zhao, S.; Zheng, M.; Sun, H.; Li, S.; Pan, Q.; Guo, Y. Construction of heterostructured g-C3N4/ZnO/cellulose and its antibacterial activity: Experimental and theoretical investigations. Dalton Trans. 2020, 49, 3723–3734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Zhang, G.; Wang, X. Synthesis of Carbon Nitride Semiconductors in Sulfur Flux for Water Photoredox Catalysis. ACS Catal. 2012, 2, 940–948. [Google Scholar] [CrossRef]
- Qin, H.; Zuo, Y.; Jin, J.; Wang, W.; Xu, Y.; Cui, L.; Dang, H. ZnO nanorod arrays grown on g-C3N4 micro-sheets for enhanced visible light photocatalytic H2 evolution. RSC Adv. 2019, 9, 24483–24488. [Google Scholar] [CrossRef] [PubMed]
- Abdel Messih, M.F.; Ahmed, M.A.; Soltan, A.; Anis, S.S. Synthesis and characterization of novel Ag/ZnO nanoparticles for photocatalytic degradation of methylene blue under UV and solar irradiation. J. Phys. Chem. Solids 2019, 135, 109086. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, W.; Huang, J.; Du, Z.; Hong, X.; Chen, X.; Hu, X.; Wang, X. Enhanced photocatalytic nitrogen fixation of Ag/B-doped g-C3N4 nanosheets by one-step in-situ decomposition-thermal polymerization method. Appl. Catal. A Gen. 2020, 601, 117647. [Google Scholar] [CrossRef]
- Qi, S.; Fei, L.; Zuo, R.; Wang, Y.; Wu, Y. Graphene nanocluster decorated niobium oxide nanofibers for visible light photocatalytic applications. J. Mater. Chem. A 2014, 2, 8190–8195. [Google Scholar] [CrossRef]
- Bozhynov, V.; Kovacs, Z.; Cisar, P.; Urban, J. Application of Visible Aquaphotomics for the Evaluation of Dissolved Chemical Concentrations in Aqueous Solutions. Photonics 2021, 8, 391. [Google Scholar] [CrossRef]
- Khademalrasool, M.; Farbod, M.; Talebzadeh, M.D. Investigation of shape effect of silver nanostructures and governing physical mechanisms on photo-activity: Zinc oxide/silver plasmonic photocatalyst. Adv. Powder Technol. 2021, 32, 1844–1857. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, Y.; Li, Z.; Yan, S.; Wang, N.; Zou, Z. High-yield synthesis of millimetre-long, semiconducting carbon nitride nanotubes with intense photoluminescence emission and reproducible photoconductivity. Nanoscale 2012, 4, 3687–3692. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, K.; Xu, R.; Yu, D.; Wang, W.; Gao, P.; Liu, B. Fabrication of BiVO4/BiPO4/GO composite photocatalytic material for the visible light-driven degradation. J. Clean. Prod. 2020, 247, 119108. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Yang, Z.; Shen, R.; Li, H.; Luo, X.; Chen, X.; Li, X. Fabricating the Robust g-C3N4 Nanosheets/Carbons/NiS Multiple Heterojunctions for Enhanced Photocatalytic H2 Generation: An Insight into the Trifunctional Roles of Nanocarbons. ACS Sustain. Chem. Eng. 2017, 5, 2224–2236. [Google Scholar] [CrossRef]
- Luo, W.; Ying, J.; Yu, S.; Yang, X.; Jia, Y.; Chen, M.; Zhang, H.; Gao, J.; Li, Y.; Mai, Y.-W.; et al. ZnS:Cu powders with strong visible-light photocatalysis and pyro-catalysis for room-temperature dye decomposition. Ceram. Int. 2020, 46, 12096–12101. [Google Scholar] [CrossRef]
- Segovia, M.; Alegría, M.; Aliaga, J.; Celedon, S.; Ballesteros, L.; Sotomayor-Torres, C.; González, G.; Benavente, E. Heterostructured 2D ZnO hybrid nanocomposites sensitized with cubic Cu2O nanoparticles for sunlight photocatalysis. J. Mater. Sci. 2019, 54, 13523–13536. [Google Scholar] [CrossRef]
- Nguyen, T.T.A.; Dao, T.C.V.; Vu, A.-T. Controlling the physical properties of Ag/ZnO/g-C3N4 nanocomposite by the calcination procedure for enhancing the photocatalytic efficiency. Ceram. Int. 2024, 50, 14292–14306. [Google Scholar] [CrossRef]
- Iqbal, S.; Ahmad, N.; Javed, M.; Qamar, M.A.; Bahadur, A.; Ali, S.; Ahmad, Z.; Irfan, R.M.; Liu, G.; Akbar, M.B.; et al. Designing highly potential photocatalytic comprising silver deposited ZnO NPs with sulfurized graphitic carbon nitride (Ag/ZnO/S-g-C3N4) ternary composite. J. Environ. Chem. Eng. 2021, 9, 104919. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Javed, M.; Hakami, O.; Irfan, R.M.; Ahmad, Z.; AlObaid, A.; Al-Anazy, M.M.; Baghdadi, H.B.; Abd-Rabboh, H.S.M.; et al. Design Ag-doped ZnO heterostructure photocatalyst with sulfurized graphitic C3N4 showing enhanced photocatalytic activity. Mater. Sci. Eng. B 2021, 272, 115320. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, X.; Liu, W.; Tan, P.; Chen, H.; Wu, L.; Ma, C.; Xiong, X.; Pan, J. Photocorrosion inhibition and high-efficiency photoactivity of porous g-C3N4/Ag2CrO4 composites by simple microemulsion-assisted co-precipitation method. Appl. Catal. B Environ. 2017, 204, 78–88. [Google Scholar] [CrossRef]
- Duan, S.-F.; Tao, C.-L.; Geng, Y.-Y.; Yao, X.-Q.; Kang, X.-W.; Su, J.-Z.; Rodríguez-Gutiérrez, I.; Kan, M.; Romero, M.; Sun, Y.; et al. Phosphorus-doped Isotype g-C3N4/g-C3N4: An Efficient Charge Transfer System for Photoelectrochemical Water Oxidation. ChemCatChem 2019, 11, 729–736. [Google Scholar] [CrossRef]













| Sr. No. | Catalyst | Morphology | Heterojunction | C (mg/L) | Light Source | T (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ag-ZnO/g-C3N4 | 0D-2D | II | 10 | 250 W Hg lamp | 60 | 100 | [55] |
| 2 | Ag/ZnO/S-g-C3N4 | 0D-2D | II | 10 | Sunlight (57–63 Klux) | 60 | 98 | [56] |
| 3 | 7Ag-ZnO/S-g-C3N4 | 0D-2D | II | 10 | Sunlight (57–63 Klux) | 40 | 97 | [57] |
| 4 | 0.05Ag-ZnO/g-C3N4 | 1D-0D-2D | Z | 20 | 300 W xenon lamp | 30 | 98.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Li, J. High-Efficiency Ag-Modified ZnO/g-C3N4 Photocatalyst with 1D-0D-2D Morphology for Methylene Blue Degradation. Molecules 2024, 29, 2182. https://doi.org/10.3390/molecules29102182
Qiu S, Li J. High-Efficiency Ag-Modified ZnO/g-C3N4 Photocatalyst with 1D-0D-2D Morphology for Methylene Blue Degradation. Molecules. 2024; 29(10):2182. https://doi.org/10.3390/molecules29102182
Chicago/Turabian StyleQiu, Shuyao, and Jin Li. 2024. "High-Efficiency Ag-Modified ZnO/g-C3N4 Photocatalyst with 1D-0D-2D Morphology for Methylene Blue Degradation" Molecules 29, no. 10: 2182. https://doi.org/10.3390/molecules29102182




