The Simultaneous Efficient Recovery of Ammonia Nitrogen and Phosphate Resources in the Form of Struvite: Optimization and Potential Applications for the Electrochemical Reduction of NO3−
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Impact of the Current Density
2.2. The Impact of the Initial pH
2.3. The Impact of Coexisting Ions in Nitrate Reduction Wastewater
2.3.1. The Effect of Nitrate
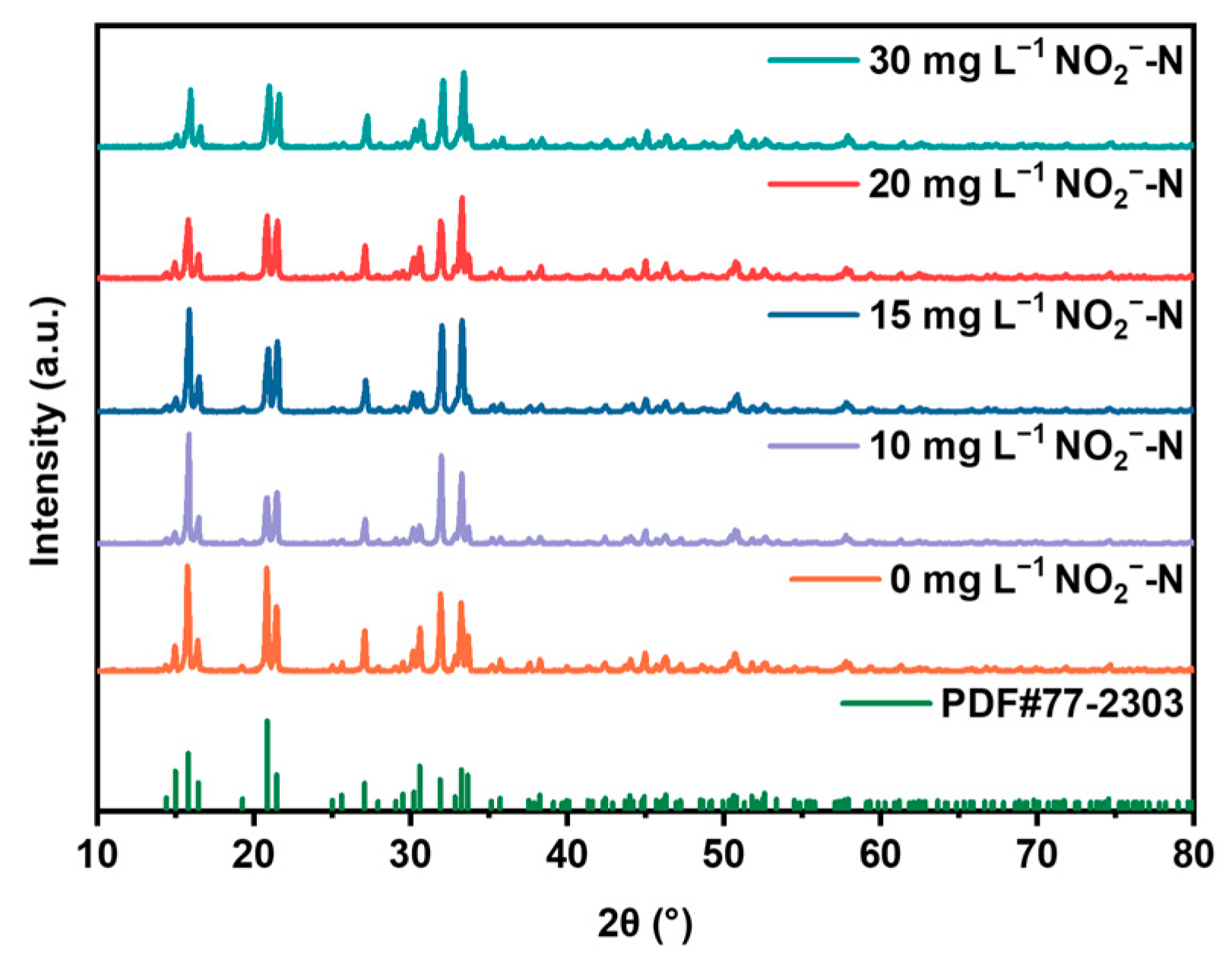
2.3.2. The Effect of Nitrite
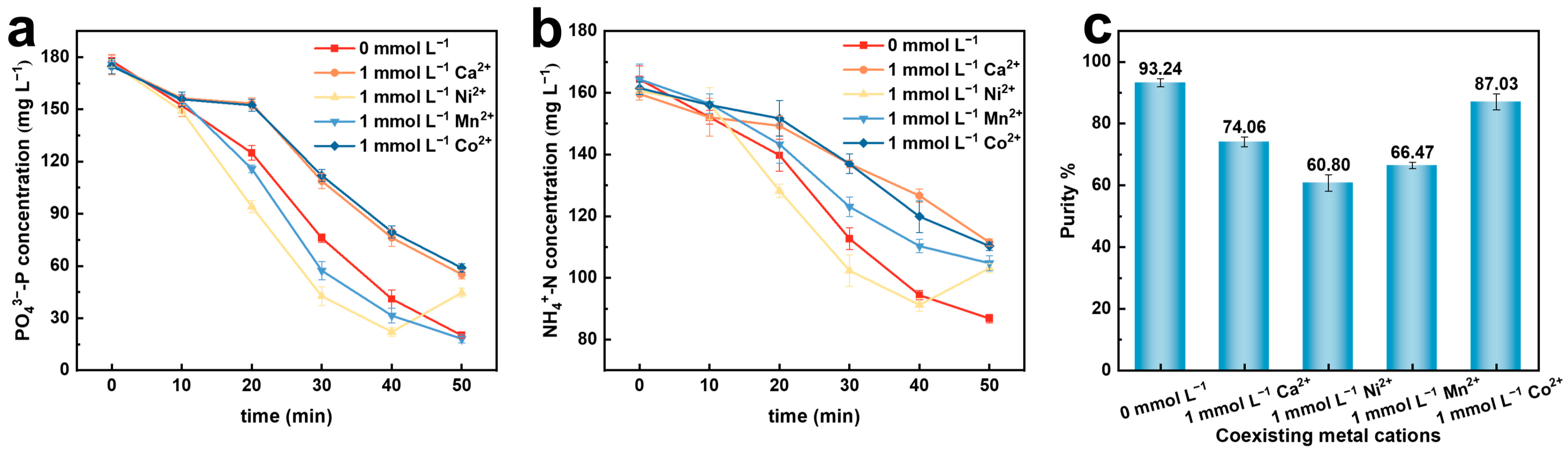
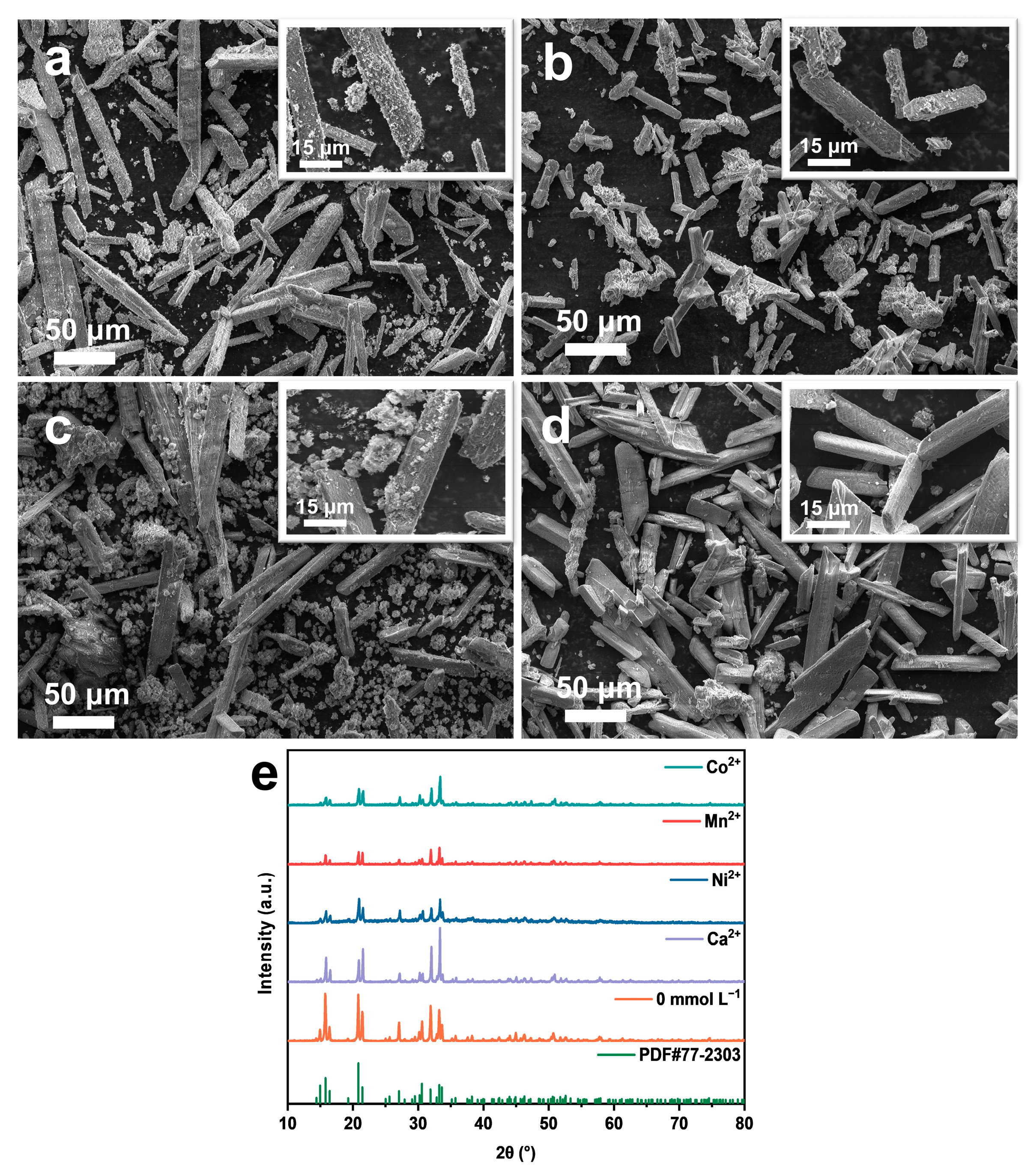
2.4. The Impact of Coexisting Metal Cations
3. Potential Implications
4. Materials and Methods
4.1. Materials
4.2. Characterization
4.3. Experimental Apparatus
4.4. Experimental Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; He, J.; Pang, H.; Jiang, P.; Qu, F.; Yu, D.; Zhang, J. New insight into electrochemical denitrification using a self-organized nanoporous VO-Co3O4/Co cathode: Plasma-assistant oxygen vacancies catalyzed efficient nitrate reduction. Sci. Total Environ. 2022, 850, 157845. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bi, J.; Huang, X.; Wang, T.; Wang, Z.; Hao, H. Bi2O3 nanosheets arrays in-situ decorated on carbon cloth for efficient electrochemical reduction of nitrate. Chemosphere 2021, 278, 130386. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Ye, J.; Wan, C.; He, G.; Chen, H. Research Progress on Cu-Based Catalysts for Electrochemical Nitrate Reduction Reaction to Ammonia. Ind. Eng. Chem. Res. 2022, 61, 14731–14746. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Wu, Z.-L.; Lin, C.-Y.; Huang, Y.-H.; Huang, C.-P. Manipulating the crystalline morphology and facet orientation of copper and copper-palladium nanocatalysts supported on stainless steel mesh with the aid of cationic surfactant to improve the electrochemical reduction of nitrate and N2 selectivity. Appl. Catal. B Environ. 2020, 273, 119053. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Y.; Wang, C.; Ling, Y.; Yu, Y.; Zhang, B. Boosting Selective Nitrate Electroreduction to Ammonium by Constructing Oxygen Vacancies in TiO2. ACS Catal. 2020, 10, 3533–3540. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Mumtarin, Z.; Rahman, M.M. Electrocatalytic reduction of hydroxylamine on copper immobilized platinum surface: Heterogeneous kinetics and sensing performance. Electrochim. Acta 2019, 318, 486–495. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Y.; Chen, J.; Zhang, W.X.; Yang, J. Electrocatalytic reduction of nitrate—A step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 2022, 51, 2710–2758. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Qin, Y.; Wang, Y.; Liu, S.; Yu, B.; Song, Y.; Wang, X.; Zhu, G. Dissimilatory nitrate/nitrite reduction to ammonium (DNRA) pathway dominates nitrate reduction processes in rhizosphere and non-rhizosphere of four fertilized farmland soil. Environ. Res. 2020, 186, 109612. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, Y.; Zhang, G.; Ruan, R.; Wang, Y.; Wu, X.; Zheng, H.; Zhang, Q.; Cao, L. New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters. World J. Microbiol. Biotechnol. 2020, 36, 144. [Google Scholar] [CrossRef]
- He, Q.; Xi, J.; Shi, M.; Feng, L.; Yan, S.; Deng, L. Developing a Vacuum-Assisted Gas-Permeable Membrane Process for Rapid Ammonia Recovery and CO2 Capture from Biogas Slurry. ACS Sustain. Chem. Eng. 2019, 8, 154–162. [Google Scholar] [CrossRef]
- Devda, V.; Chaudhary, K.; Varjani, S.; Pathak, B.; Patel, A.K.; Singhania, R.R.; Taherzadeh, M.J.; Ngo, H.H.; Wong, J.W.C.; Guo, W.; et al. Recovery of resources from industrial wastewater employing electrochemical technologies: Status, advancements and perspectives. Bioengineered 2021, 12, 4697–4718. [Google Scholar] [CrossRef]
- Hug, A.; Udert, K.M. Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 2013, 47, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Guo, G.; Zhang, P.; Zhang, D.; Liu, J.; Tang, S. Feasibility of physicochemical recovery of nutrients from swine wastewater: Evaluation of three kinds of magnesium sources. J. Taiwan Inst. Chem. Eng. 2017, 70, 209–218. [Google Scholar] [CrossRef]
- Kruk, D.J.; Elektorowicz, M.; Oleszkiewicz, J.A. Struvite precipitation and phosphorus removal using magnesium sacrificial anode. Chemosphere 2014, 101, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-D.; Lee, S.-I. Struvite recovery from swine wastewater and its assessment as a fertilizer. Environ. Eng. Res. 2016, 21, 29–35. [Google Scholar] [CrossRef]
- Wang, L.; Gu, K.; Zhang, Y.; Sun, J.; Gu, Z.; Zhao, B.; Hu, C. Enhanced struvite generation and separation by magnesium anode electrolysis coupled with cathode electrodeposition. Sci. Total Environ. 2022, 804, 150101. [Google Scholar] [CrossRef]
- Lin, X.; Han, Z.; Yu, H.; Ye, Z.; Zhu, S.; Zhu, J. Struvite precipitation from biogas digestion slurry using a two-chamber electrolysis cell with a magnesium anode. J. Clean. Prod. 2018, 174, 1598–1607. [Google Scholar] [CrossRef]
- Li, Y.-F.; Wang, C.-W.; Chen, C.-K.; Chen, M.-W.; Fang, Y.-C.; Lo, S.-L. Recovering Phosphorus from Human Urine by Electrochemical Precipitation Using Magnesium Alloy Tailings as Electrodes: Lab Scale and Pilot Scale Studies. J. Environ. Eng. 2024, 150, 05024002. [Google Scholar] [CrossRef]
- Bagastyo, A.Y.; Anggrainy, A.D.; Khoiruddin, K.; Ursada, R.; Warmadewanthi, I.; Wenten, I.G. Electrochemically-driven struvite recovery: Prospect and challenges for the application of magnesium sacrificial anode. Sep. Purif. Technol. 2022, 288, 120653. [Google Scholar] [CrossRef]
- Ma, N.; Rouff, A.A.; Phillips, B.L. A 31P NMR and TG/DSC-FTIR Investigation of the Influence of Initial pH on Phosphorus Recovery as Struvite. ACS Sustain. Chem. Eng. 2014, 2, 816–822. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- González-Morales, C.; Fernández, B.; Molina, F.J.; Naranjo-Fernández, D.; Matamoros-Veloza, A.; Camargo-Valero, M.A. Influence of pH and Temperature on Struvite Purity and Recovery from Anaerobic Digestate. Sustainability 2021, 13, 10730. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.; Guo, L.; Nie, J.; Li, C. Removal and recovery of phosphate anion as struvite from wastewater. Clean Technol. Environ. Policy 2018, 20, 2375–2380. [Google Scholar] [CrossRef]
- Liu, X.; Wen, G.; Hu, Z.; Wang, J. Coupling effects of pH and Mg/P ratio on P recovery from anaerobic digester supernatant by struvite formation. J. Clean. Prod. 2018, 198, 633–641. [Google Scholar] [CrossRef]
- Wang, C.C.; Hao, X.D.; Guo, G.S.; van Loosdrecht, M.C.M. Formation of pure struvite at neutral pH by electrochemical deposition. Chem. Eng. J. 2010, 159, 280–283. [Google Scholar] [CrossRef]
- Perwitasari, D.S.; Muryanto, S.; Jamari, J.; Bayuseno, A.P. Kinetics and morphology analysis of struvite precipitated from aqueous solution under the influence of heavy metals: Cu2+, Pb2+, Zn2+. J. Environ. Chem. Eng. 2018, 6, 37–43. [Google Scholar] [CrossRef]
- Liu, Y.; Kwag, J.-H.; Kim, J.-H.; Ra, C. Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination 2011, 277, 364–369. [Google Scholar] [CrossRef]
- Masindi, V.; Fosso-Kankeu, E.; Mamakoa, E.; Nkambule, T.T.I.; Mamba, B.B.; Naushad, M.; Pandey, S. Emerging remediation potentiality of struvite developed from municipal wastewater for the treatment of acid mine drainage. Environ. Res. 2022, 210, 112944. [Google Scholar] [CrossRef]
- Yan, H.; Shih, K. Effects of calcium and ferric ions on struvite precipitation: A new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous recovery of ammonium, potassium and magnesium from produced water by struvite precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Hao, X.D.; Wang, C.C.; Lan, L.; van Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2 +. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef]
- Vol’khin, V.V.; Kazakov, D.A.; Leont’eva, G.V.; Andreeva, Y.V.; Nosenko, E.A.; Siluyanova, M.Y. Synthesis of struvite (MgNH4PO4·6H2O) and its use for sorption of nickel ions. Russ. J. Appl. Chem. 2015, 88, 1986–1996. [Google Scholar] [CrossRef]
- Nandre, V.; Battu, S.; Itagi, M.; Patil, S.; Kumbhar, N.; Gejji, S.P.; Malkhede, D.; Thotiyl, M.O.; Kodam, K. Siderophore mediated in vitro synthesis of electrocatalytic nanocrystallite struvite variants for highly efficient and durable hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 51, 828–836. [Google Scholar] [CrossRef]
- Sun, H.; Mohammed, A.N.; Liu, Y. Phosphorus recovery from source-diverted blackwater through struvite precipitation. Sci. Total Environ. 2020, 743, 140747. [Google Scholar] [CrossRef] [PubMed]
- Goswami, O.; Rouff, A.A. Interaction of divalent metals with struvite: Sorption, reversibility, and implications for mineral recovery from wastes. Environ. Technol. 2023, 44, 2315–2326. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Z.; Peng, C.; Chai, L.-Y.; Kuroda, K.; Okido, M.; Song, Y.-X. New insights into the interaction between heavy metals and struvite: Struvite as platform for heterogeneous nucleation of heavy metal hydroxide. Chem. Eng. J. 2019, 365, 60–69. [Google Scholar] [CrossRef]
- Hövelmann, J.; Stawski, T.M.; Freeman, H.M.; Besselink, R.; Mayanna, S.; Perez, J.P.H.; Hondow, N.S.; Benning, L.G. Struvite Crystallisation and the Effect of Co2+ Ions. Minerals 2019, 9, 503. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Ilhan, F.; Kocak, E.; Akbin, H.M. Feasibility of struvite recovery process for fertilizer industry: A study of financial and economic analysis. J. Clean. Prod. 2017, 152, 88–102. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Bi, J.; Sun, M.; Wang, S.; Guo, X.; Li, F.; Liu, J.; Zhao, Y. The Simultaneous Efficient Recovery of Ammonia Nitrogen and Phosphate Resources in the Form of Struvite: Optimization and Potential Applications for the Electrochemical Reduction of NO3−. Molecules 2024, 29, 2185. https://doi.org/10.3390/molecules29102185
Li L, Bi J, Sun M, Wang S, Guo X, Li F, Liu J, Zhao Y. The Simultaneous Efficient Recovery of Ammonia Nitrogen and Phosphate Resources in the Form of Struvite: Optimization and Potential Applications for the Electrochemical Reduction of NO3−. Molecules. 2024; 29(10):2185. https://doi.org/10.3390/molecules29102185
Chicago/Turabian StyleLi, Liping, Jingtao Bi, Mengmeng Sun, Shizhao Wang, Xiaofu Guo, Fei Li, Jie Liu, and Yingying Zhao. 2024. "The Simultaneous Efficient Recovery of Ammonia Nitrogen and Phosphate Resources in the Form of Struvite: Optimization and Potential Applications for the Electrochemical Reduction of NO3−" Molecules 29, no. 10: 2185. https://doi.org/10.3390/molecules29102185





