Determination of Sulfites in Dried Fruits by Paper Spray Ionization Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
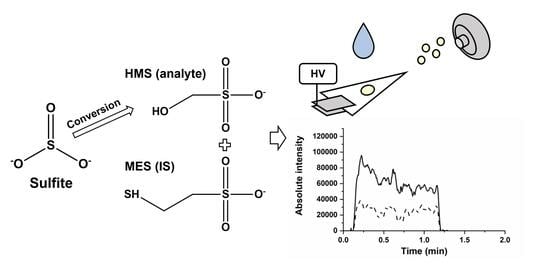
2.1. Alternative Internal Standard for PSI and LC-MS/MS Analysis of Sulfite
2.2. PSI and LC-MS/MS Analysis of Sulfite with Dried Fruits
3. Materials and Methods
3.1. Materials
3.2. Standard Preparation
3.3. Sample Preparation for Dried Fruits
3.4. Liquid Chromatography (LC)-Tandem Mass Spectrometry (MS/MS)
3.5. Paper Spray Ionization (PSI)-Tandem Mass Spectrometry (MS/MS)
3.6. Quantitation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robbins, K.S.; Shah, R.; MacMahon, S.; de Jager, L.S. Development of a Liquid Chromatography–Tandem Mass Spectrometry Method for the Determination of Sulfite in Food. J. Agric. Food Chem. 2015, 63, 5126–5132. [Google Scholar] [CrossRef] [PubMed]
- Carlos, K.S.; Treblin, M.; de Jager, L.S. Comparison and optimization of three commercial methods with an LC–MS/MS method for the determination of sulfites in food and beverages. Food Chem. 2019, 286, 537–540. [Google Scholar] [CrossRef]
- Perfetti, G.A.; Diachenko, G.W. Determination of Sulfite in Dried Garlic by Reversed-Phase Ion-Pairing Liquid Chromatography with Post-Column Detection. J. AOAC Int. 2003, 86, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Çetintaş, G. Determination of Sulfite in Water and Dried Fruit Samples by Dispersive Liquid–Liquid Microextraction Combined with UV–Vis Fiber Optic Linear Array Spectrophotometry. Food Anal. Methods 2012, 5, 1362–1367. [Google Scholar] [CrossRef]
- Temel, N.K. Ultrasound assisted-cloud point extraction coupled with spectrophotometry for determination of low levels of formaldehyde from milk-based products. J. Food Compos. Anal. 2024, 126, 105919. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Cheng, N.; Li, D.; Qi, X.; Sun, Z. Determination of free sulfite in food samples with a novel fluorescent probe as gas-diffusion microextraction acceptor phase. Microchem. J. 2024, 199, 110211. [Google Scholar] [CrossRef]
- Huang, L.; Lai, L.; Zhang, X.; Lin, S.; Jin, G.; Li, D. Practical assay for determining residual sulfite of the wine in rapid detection or quantitative analysis. LWT—Food Sci. Technol. 2023, 189, 115503. [Google Scholar] [CrossRef]
- Lowinsohn, D.; Bertotti, M. Determination of sulphite in wine by coulometric titration. Food Addit. Contam. 2001, 18, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.; Davis, J.; Livingstone, C.; Wain, A.J.; Compton, R.G. Electroanalytical methods for the determination of sulfite in food and beverages. TrAC Trends Anal. Chem. 2006, 25, 589–598. [Google Scholar] [CrossRef]
- Montes, C.; Vélez, J.H.; Ramírez, G.; Isaacs, M.; Arce, R.; Aguirre, M.J. Critical Comparison between Modified Monier-Williams and Electrochemical Methods to Determine Sulfite in Aqueous Solutions. Sci. World J. 2012, 2012, 168148. [Google Scholar] [CrossRef]
- Warner, C.R.; Daniels, D.H.; Fitzgerald, M.C.; Joe Jr, F.L.; Diachenko, G.W. Determination of free and reversibly bound sulphite in foods by reverse-phase, ion-pairing high-performance liquid chromatography. Food Addit. Contam. 1990, 7, 575–581. [Google Scholar] [CrossRef]
- Iammarino, M.; Di Taranto, A.; Muscarella, M.; Nardiello, D.; Palermo, C.; Centonze, D. Development of a new analytical method for the determination of sulfites in fresh meats and shrimps by ion-exchange chromatography with conductivity detection. Anal. Chim. Acta 2010, 672, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Carlos, K.S.; de Jager, L.S. Determination of Sulfite in Food by Liquid Chromatography Tandem Mass Spectrometry: Collaborative Study. J. AOAC Int. 2017, 100, 1785–1794. [Google Scholar] [CrossRef]
- Warner, C.R.; Daniels, D.H.; Pratt, D.E.; Joe, F.L., Jr.; Fazio, T.; Diachenko, G.W. Sulphite stabilization and high-performance liquid chromatographic determination: A reference method for free and reversibly bound sulphite in food. Food Addit. Contam. 1987, 4, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Manicke, N.E.; Lin, J.-M.; Cooks, R.G.; Ouyang, Z. Development, Characterization, and Application of Paper Spray Ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef]
- Espy, R.D.; Muliadi, A.R.; Ouyang, Z.; Cooks, R.G. Spray mechanism in paper spray ionization. Int. J. Mass Spectrom. 2012, 325–327, 167–171. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Cooks, R.G.; Ouyang, Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angew. Chem. Int. Ed. 2010, 49, 877–880. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, H.; Liu, J.; Zhang, Z.; McLuckey, M.N.; Ouyang, Z. Analysis of Biological Samples Using Paper Spray Mass Spectrometry: An Investigation of Impacts by the Substrates, Solvents and Elution Methods. Chromatographia 2013, 76, 1339–1346. [Google Scholar] [CrossRef]
- Evard, H.; Kruve, A.; Lõhmus, R.; Leito, I. Paper spray ionization mass spectrometry: Study of a method for fast-screening analysis of pesticides in fruits and vegetables. J. Food Compos. Anal. 2015, 41, 221–225. [Google Scholar] [CrossRef]
- Kim, P.; Cha, S. Paper cone spray ionization mass spectrometry (PCSI MS) for simple and rapid analysis of raw solid samples. Analyst 2015, 140, 5868–5872. [Google Scholar] [CrossRef]
- Pereira, I.; Rodrigues, S.R.M.; de Carvalho, T.C.; Carvalho, V.V.; Lobón, G.S.; Bassane, J.F.P.; Domingos, E.; Romão, W.; Augusti, R.; Vaz, B.G. Rapid screening of agrochemicals by paper spray ionization and leaf spray mass spectrometry: Which technique is more appropriate? Anal. Methods 2016, 8, 6023–6029. [Google Scholar] [CrossRef]
- Beneito-Cambra, M.; Gilbert-López, B.; Moreno-González, D.; Bouza, M.; Franzke, J.; García-Reyes, J.F.; Molina-Díaz, A. Ambient (desorption/ionization) mass spectrometry methods for pesticide testing in food: A review. Anal. Methods 2020, 12, 4831–4852. [Google Scholar] [CrossRef]
- Cheng, S.C.; Lee, R.H.; Jeng, J.Y.; Lee, C.W.; Shiea, J. Fast screening of trace multiresidue pesticides on fruit and vegetable surfaces using ambient ionization tandem mass spectrometry. Anal. Chim. Acta 2020, 1102, 63–71. [Google Scholar] [CrossRef]
- Ramalho, R.R.F.; da Silva, L.C.; Maciel, L.I.L.; Pereira, I.; Nascimento, A.d.R.; Simas, R.C.; Vaz, B.G. Directly transferring pepper constituents to triangular papers for pungency determination by paper spray ionization mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 5389–5396. [Google Scholar] [CrossRef]
- Haug, M.T.; King, E.S.; Heymann, H.; Crisosto, C.H. Sensory Profiles for Dried Fig (Ficus carica L.) Cultivars Commercially Grown and Processed in California. J. Food Sci. 2013, 78, S1273–S1281. [Google Scholar] [CrossRef]




| Method | 16 ppm Na2SO3 | 32 ppm Na2SO3 | 80 ppm Na2SO3 |
|---|---|---|---|
| LC-MS/MS | 91 ± 3 | 99 ± 4 | 106 ± 4 |
| PSI-MS/MS | 88 ± 9 | 96 ± 10 | 109 ± 8 |
| Method | Apricot | Mango | Tomato | Fig 1 |
|---|---|---|---|---|
| LC-MS/MS | 463 ± 27 | 55 ± 3 | 224 ± 8 | ND 2 |
| PSI-MS/MS | 482 ± 44 | 42 ± 6 | 213 ± 6 | ND |
| Compound ID | Q1 m/z | Q3 m/z | CE (V) | TL (V) |
|---|---|---|---|---|
| HMS | 111 | 81 | 27 | 25 |
| HMS | 111 | 80 | 14 | 25 |
| MES (IS) | 141 | 81 | 17 | 42 |
| MES (IS) | 141 | 80 | 31 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Ro, H.; Hwang, S.; Lee, M.; Kim, H.; Heo, J.; Cha, S. Determination of Sulfites in Dried Fruits by Paper Spray Ionization Tandem Mass Spectrometry. Molecules 2024, 29, 2192. https://doi.org/10.3390/molecules29102192
Lee D, Ro H, Hwang S, Lee M, Kim H, Heo J, Cha S. Determination of Sulfites in Dried Fruits by Paper Spray Ionization Tandem Mass Spectrometry. Molecules. 2024; 29(10):2192. https://doi.org/10.3390/molecules29102192
Chicago/Turabian StyleLee, Donghoon, Heejin Ro, Seoyoung Hwang, Minkyu Lee, Hyebeen Kim, Jaeyoung Heo, and Sangwon Cha. 2024. "Determination of Sulfites in Dried Fruits by Paper Spray Ionization Tandem Mass Spectrometry" Molecules 29, no. 10: 2192. https://doi.org/10.3390/molecules29102192