NGAL in the Development of Acute Kidney Injury in a Murine Model of Remote Ischaemic Preconditioning and Liver Ischaemia Reperfusion
Abstract
:1. Introduction
2. Results
2.1. The Mouse Model of Liver Ischaemia Reperfusion Was Associated with Liver and Renal Injury
2.2. Liver and Renal Injury Are Associated with Upregulation of Serum NGAL
2.3. With Liver IR Injury and AKI, NGAL mRNA Upregulation Is Seen in the Liver but Not the Kidney. The Liver NGAL mRNA Was Reduced by Pre-Treatment with RIPC
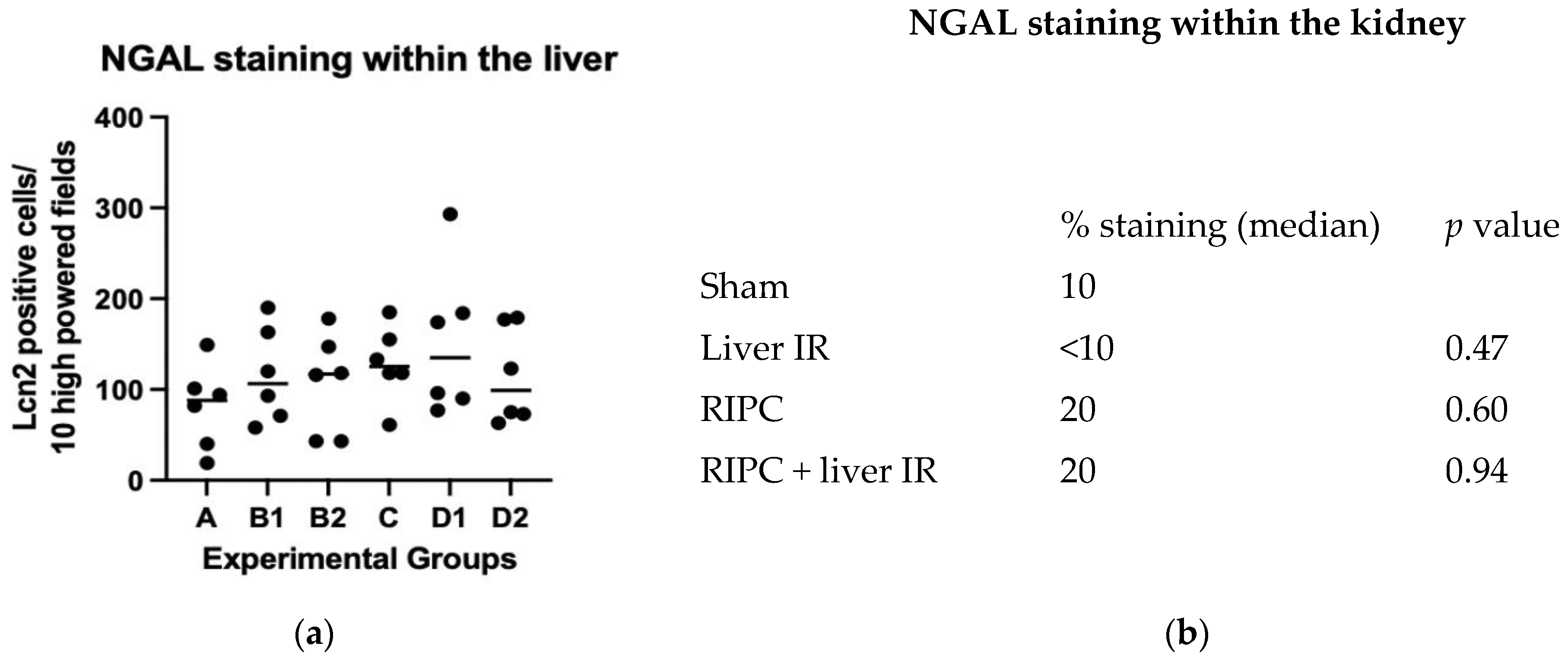
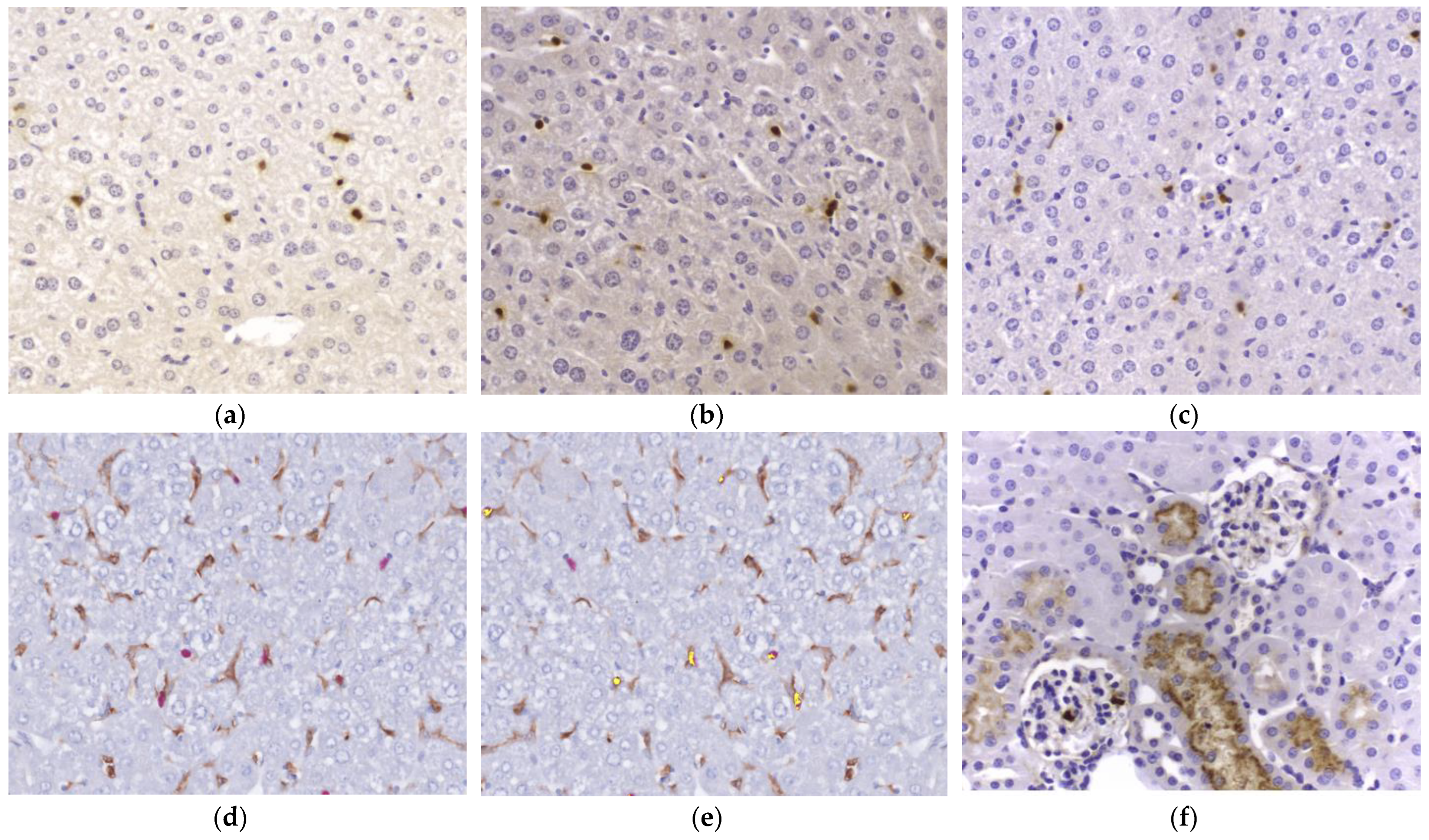
2.4. NGAL Expression by IHC in the Liver and Kidney Compared with Plasma NGAL Concentration
2.5. In the Liver, NGAL Demonstrates Partial Co-Localisation with F4/80
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Operative Procedure
4.3. Sham Procedures/Controls
4.4. Remote Ischaemic-Preconditioning (RIPC)
4.5. Liver Ischaemia Reperfusion (IR)
4.6. Remote Ischaemic Pre-Conditioning + Liver Ischaemia Reperfusion (RIPC + Liver IR)
4.7. Quantification of Liver and Kidney Injury
4.8. Plasma NGAL
4.9. NGAL Immunohistochemistry of Liver and Kidney Specimens
4.10. Multiplex F4/80—NGAL Immunohistochemistry
4.11. Interpretation of IHC
4.12. Interpretation of Multiplex F4/80-NGAL IHC
4.13. DNA/RNA Oxidative Damage Immunohistochemistry
4.14. NGAL qPCR
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
Appendix C
| Primer Pair | Primer Sequence 5′-3′ | Efficiency | Comments |
|---|---|---|---|
| NGAL/1 | f-ATGTCACCTCCATCCTGGTCA r-ACAGCTCCTGGTTCTTCCATACAG | 80.0% | Dimerization |
| NGAL/2 * | fGGGAAATATGCACAGGTATCCTC r-CATGGCGAACTGGTTGTAGTC | 103.6% | |
| NGAL/3 | f-TGGCCCTGAGTGTCATGTG r-CTCTTGTAGCTCATAGATGGTGC | 92.5% | Primer pair does not span intron |
| NGAL/4 | f-GCAGGTGGTACGTTGTGGG r-CTCTTGTAGCTCATAGATGGTGC | 108.0% | Primer pair does not span intron |
| Normalization Gene | Primer Sequence 5′-3′ | Efficiency | Comments |
|---|---|---|---|
| CCT5 | f-CTGGGCTCCAAAGTGATTAACA r-TCTCTCCGCTCCATATCTGCC | 82.9% | |
| P4hb * | f-ACCTGCTGGTGGAGTTCTATGC r-ATTGTGGGGTAGCCACGGAC | 93.8% | |
| Cali * | f-TTCTTGGACGGAGATGCCTG r-GGCCCTTATTGCTGAAGGGT | 87.9% | |
| GAPDH | f-GCAATTATTCCCCATGAACG r-GGCCTCACTAAACCATCCAA | 38.9% | Primers from literature |
References
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, E.; Nieß, H.; Khaled, N.B.; Bösch, F.; Guba, M.; Werner, J.; Angele, M.; Chaudry, I.H. Molecular Mechanisms of Ischaemia-Reperfusion Injury and Regeneration in the Liver-Shock and Surgery-Associated Changes. Int. J. Mol. Sci. 2022, 23, 12942. [Google Scholar] [CrossRef] [PubMed]
- Rampes, S.; Ma, D. Hepatic Ischemia-Reperfusion Injury in Liver Transplant Setting: Mechanisms and Protective Strategies. J. Biomed. Res. 2019, 33, 221–234. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.H.C.; Marques, P.E.; Proost, P.; Teixeira, M.M.M. Neutrophils: A Cornerstone of Liver Ischemia and Reperfusion Injury. Lab. Investig. 2018, 98, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Capuzzimati, M.; Hough, O.; Liu, M. Cell Death and Ischemia-Reperfusion Injury in Lung Transplantation. J. Hearth Lung Transpl. 2022, 41, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Saidi, R.F.; Kenari, S.K.H. Liver Ischemia/Reperfusion Injury: An Overview. J. Investig. Surg. 2014, 27, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, K.E.F.; Hunt, A.C.; Palmer, D.B.; Badrick, F.E.; Morris, A.M.; Mitra, S.K.; Peacock, J.H.; Immelman, E.J.; Riddell, A.G. Hypothermic Low Flow Liver Perfusion as a Means of Porcine Hepatic Storage for Six Hours. Br. J. Surg. 1968, 55, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.M.; Gores, G.J.; Ludwig, J.; Krom, R.A.F. UW Solution Protects against Reperfusion Injury by Inhibiting Lipid Peroxidation. Transpl. Proc. 1991, 23, 1552–1553. [Google Scholar]
- Hann, A.; Lembach, H.; Carvalheiro, A.; Boteon, Y.; McKay, S.; Kadam, P.; Dissanayake, B.; Tilakaratne, S.; Alzoubi, M.; Bartlett, D.; et al. Normothermic machine perfusion of marginal liver allografts is associated with a low incidence of post reperfusion syndrome in high risk recipients. Transplantation 2020, 104, S60. [Google Scholar] [CrossRef]
- Clavien, P.A.; Yadav, S.; Sindram, D.; Bentley, R.C. Protective Effects of Ischemic Preconditioning for Liver Resection Performed under Inflow Occlusion in Humans. Ann. Surg. 2000, 232, 155–162. [Google Scholar] [CrossRef]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional Ischemic “preconditioning” Protects Remote Virgin Myocardium from Subsequent Sustained Coronary Occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef]
- Mukai, A.; Suehiro, K.; Kimura, A.; Fujimoto, Y.; Funao, T.; Mori, T.; Nishikawa, K. Protective Effects of Remote Ischemic Preconditioning against Spinal Cord Ischemia–Reperfusion Injury in Rats. J. Thorac. Cardiovasc. Surg. 2022, 163, e137–e156. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, J.; Feng, B.; Gui, L.; Chen, Q.; Zhang, B.; Tang, J.; Li, X. Remote Ischemic Preconditioning Promotes Early Liver Cell Proliferation in a Rat Model of Small-for-Size Liver Transplantation. J. Surg. Res. 2013, 179, e245–e253. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, C.; Zeng, X.; Sun, C. The Hepatoprotective Effect from Ischemia-Reperfusion Injury of Remote Ischemic Preconditioning in the Liver Related Surgery: A Meta-Analysis. ANZ J. Surg. 2022, 92, 1332–1337. [Google Scholar] [CrossRef]
- Chu, Y.-J.; Li, X.-W.; Wang, P.-H.; Xu, J.; Sun, H.-J.; Ding, M.; Jiao, J.; Ji, X.-Y.; Feng, S. Clinical Outcomes of Toe Amputation in Patients with Type 2 Diabetes in Tianjin, China. Int. Wound J. 2016, 13, 175–181. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, J.; Jia, Y.; Xue, M. Remote Ischemic Preconditioning Protects against Cerebral Ischemia Injury in Rats by Upregulating MiR-204-5p and Activating the PINK1/Parkin Signaling Pathway. Metab. Brain Dis. 2022, 37, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amara, M.; Yang, S.Y.; Quaglia, A.; Rowley, P.; Tapuria, N.; Seifalian, A.M.; Fuller, B.J.; Davidson, B.R. Effect of Remote Ischemic Preconditioning on Liver Ischemia/Reperfusion Injury Using a New Mouse Model. Liver Transpl. 2011, 17, 70–82. [Google Scholar] [CrossRef]
- Stankiewicz, R.; Grąt, M. Direct, Remote and Combined Ischemic Conditioning in Liver Surgery. World J. Hepatol. 2021, 13, 533. [Google Scholar] [CrossRef]
- Belon, A.R.; Tannuri, A.C.A.; de Albuquerque Rangel Moreira, D.; Figueiredo, J.L.; da Silva, A.M.; Serafini, S.; Guimarães, R.R.; Faria, C.S.; de Alexandre, A.S.; Gonçalves, J.O.; et al. Impact of Three Methods of Ischemic Preconditioning on Ischemia-Reperfusion Injury in a Pig Model of Liver Transplantation. J. Investig. Surg. 2022, 35, 900–909. [Google Scholar] [CrossRef]
- Kyrychenko, M.I.; Biliaiev, A.V.; Mazur, A.P. Remote donor preconditioning for increasing transplant survival in the recipient’s body during the kidney transplantation from the living-related donor. Wiad. Lek. 2022, 75, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, W.; Pommergaard, H.C.; Rasmussen, A. Remote Ischemic Preconditioning of Transplant Recipients to Reduce Graft Ischemia and Reperfusion Injuries: A Systematic Review. Transpl. Rev. 2018, 32, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Robertson, F.P.; Goswami, R.; Wright, G.P.; Imber, C.; Sharma, D.; Malago, M.; Fuller, B.J.; Davidson, B.R. Remote Ischaemic Preconditioning in Orthotopic Liver Transplantation (RIPCOLT Trial): A Pilot Randomized Controlled Feasibility Study. HPB 2017, 19, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.M.H.; Kharbanda, R.K.; Konstantinov, I.E.; Shimizu, M.; Frndova, H.; Li, J.; Holtby, H.M.; Cox, P.N.; Smallhorn, J.F.; Van Arsdell, G.S.; et al. Randomized Controlled Trial of the Effects of Remote Ischemic Preconditioning on Children Undergoing Cardiac Surgery. First Clinical Application in Humans. J. Am. Coll. Cardiol. 2006, 47, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Jahangirifard, A.; Ahmadi, Z.H.; Khalili, N.; Naghashzadeh, F.; Afshar, A.; Amiri, A.; Dalili, N. Early Post-Operative Acute Kidney Injury after Cardiac Transplantation: Incidence and Predictive Factors. Clin. Transpl. 2021, 35, e14420. [Google Scholar] [CrossRef]
- Jochmans, I.; Meurisse, N.; Neyrinck, A.; Verhaegen, M.; Monbaliu, D.; Pirenne, J. Hepatic Ischemia/Reperfusion Injury Associates with Acute Kidney Injury in Liver Transplantation: Prospective Cohort Study. Liver Transpl. 2017, 23, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Planinsic, R.; Boucek, C.; Sakai, T.; Chang, C.C.H.; Kellum, J.A. Acute Kidney Injury Following Orthotopic Liver Transplantation: Incidence, Risk Factors, and Effects on Patient and Graft Outcomes. Br. J. Anaesth. 2015, 114, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Davidson, B.R.; Mallett, S.V. Early Acute Kidney Injury after Liver Transplantation: Predisposing Factors and Clinical Implications. World J. Hepatol. 2017, 9, 823. [Google Scholar] [CrossRef]
- Guo, D.; Wang, H.; Lai, X.; Li, J.; Xie, D.; Zhen, L.; Jiang, C.; Li, M.; Liu, X. Development and Validation of a Nomogram for Predicting Acute Kidney Injury after Orthotopic Liver Transplantation. Ren. Fail. 2021, 43, 1588–1600. [Google Scholar] [CrossRef]
- Trinh, E.; Alam, A.; Tchervenkov, J.; Cantarovich, M. Impact of Acute Kidney Injury Following Liver Transplantation on Long-Term Outcomes. Clin. Transpl. 2017, 31, e12863. [Google Scholar] [CrossRef]
- Albert, C.; Zapf, A.; Haase, M.; Röver, C.; Pickering, J.W.; Albert, A.; Bellomo, R.; Breidthardt, T.; Camou, F.; Chen, Z.; et al. Neutrophil Gelatinase-Associated Lipocalin Measured on Clinical Laboratory Platforms for the Prediction of Acute Kidney Injury and the Associated Need for Dialysis Therapy: A Systematic Review and Meta-Analysis. Am. J. Kidney Dis. 2020, 76, 826–841. [Google Scholar] [CrossRef]
- Antonelli, A.; Allinovi, M.; Cocci, A.; Russo, G.I.; Schiavina, R.; Rocco, B.; Giovannalberto, P.; Celia, A.; Galfano, A.; Varca, V.; et al. The Predictive Role of Biomarkers for the Detection of Acute Kidney Injury After Partial or Radical Nephrectomy: A Systematic Review of the Literature. Eur. Urol. Focus 2020, 6, 344–353. [Google Scholar] [CrossRef]
- Bao, G.; Clifton, M.; Hoette, T.M.; Mori, K.; Deng, S.X.; Qiu, A.; Viltard, M.; Williams, D.; Paragas, N.; Leete, T.; et al. Iron Traffics in Circulation Bound to a Siderocalin (Ngal)-Catechol Complex. Nat. Chem. Biol. 2010, 6, 602–609. [Google Scholar] [CrossRef]
- Nasioudis, D.; Witkin, S.S. Neutrophil Gelatinase-Associated Lipocalin and Innate Immune Responses to Bacterial Infections. Med. Microbiol. Immunol. 2015, 204, 471–479. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The Multifaceted Roles of Neutrophil Gelatinase Associated Lipocalin (NGAL) in Inflammation and Cancer. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 129–169. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M. Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Acute Kidney Injury—Where Do We Stand Today? Nephrol. Dial. Transpl. 2011, 26, 762–764. [Google Scholar] [CrossRef]
- Robertson, F.P.; Yeung, A.C.; Male, V.; Rahman, S.; Mallett, S.; Fuller, B.J.; Davidson, B.R. Urinary Neutrophil Gelatinase Associated Lipocalins (NGALs) Predict Acute Kidney Injury Post Liver Transplant. HPB 2019, 21, 473–481. [Google Scholar] [CrossRef]
- Passov, A.; Ilmakunnas, M.; Pihlajoki, M.; Hermunen, K.; Lempinen, M.; Helanterä, I.; Kailari, V.; Heikinheimo, M.; Andersson, S.; Pesonen, E. Neutrophil Gelatinase-Associated Lipocalin Does Not Originate from the Kidney during Reperfusion in Clinical Renal Transplantation. Intensive Care Med. Exp. 2021, 9, 56. [Google Scholar] [CrossRef]
- Lee, S.A.; Noel, S.; Kurzhagen, J.T.; Sadasivam, M.; Pierorazio, P.M.; Arend, L.J.; Hamad, A.R.; Rabb, H. CD4+ T Cell–Derived NGAL Modifies the Outcome of Ischemic Acute Kidney Injury. J. Immunol. 2020, 204, 586–595. [Google Scholar] [CrossRef]
- Konstantinov, I.E.; Arab, S.; Li, J.; Coles, J.G.; Boscarino, C.; Mori, A.; Cukerman, E.; Dawood, F.; Cheung, M.M.H.; Shimizu, M.; et al. The Remote Ischemic Preconditioning Stimulus Modifies Gene Expression in Mouse Myocardium. J. Thorac. Cardiovasc. Surg. 2005, 130, 1326–1332. [Google Scholar] [CrossRef]
- de Haan, J.E.; Hoorn, E.J.; de Geus, H.R.H. Acute Kidney Injury after Liver Transplantation: Recent Insights and Future Perspectives. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Park, S.W.; Kim, M.; D’Agati, V.D. Acute Kidney Injury after Hepatic Ischemia and Reperfusion Injury in Mice. Lab. Investig. 2009, 89, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.Y.; Chen, F.; He, Y.; Wu, L.J.; Wang, L.Q.; Zhu, S.M.; Zheng, S. Sen Intrarenal Resistance Index for the Assessment of Acute Renal Injury in a Rat Liver Transplantation Model. BMC Nephrol. 2013, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Chen, S.W.C.; Kim, M.; D’Agati, V.D.; Lee, H.T. Human Heat Shock Protein 27-Overexpressing Mice Are Protected against Acute Kidney Injury after Hepatic Ischemia and Reperfusion. Am. J. Physiol. Ren. Physiol. 2009, 87, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.M.; Frostad, K.B. Acute Hepatic Ischemic-Reperfusion Injury Induces a Renal Cortical “Stress Response,” Renal “Cytoresistance,” and an Endotoxin Hyperresponsive State. Am. J. Physiol. Ren. Physiol. 2014, 307, F856–F868. [Google Scholar] [CrossRef] [PubMed]
- Skrypnyk, N.I.; Gist, K.M.; Okamura, K.; Montford, J.R.; You, Z.; Yang, H.; Moldovan, R.; Bodoni, E.; Blaine, J.T.; Edelstein, C.L.; et al. IL-6-Mediated Hepatocyte Production Is the Primary Source of Plasma and Urine Neutrophil Gelatinase–Associated Lipocalin during Acute Kidney Injury. Kidney Int. 2020, 97, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Borkham-Kamphorst, E.; van de Leur, E.; Zimmermann, H.W.; Karlmark, K.R.; Tihaa, L.; Haas, U.; Tacke, F.; Berger, T.; Mak, T.W.; Weiskirchen, R. Protective Effects of Lipocalin-2 (LCN2) in Acute Liver Injury Suggest a Novel Function in Liver Homeostasis. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, D.; Cai, Y.; Liu, Y.; Xu, M.; Xiang, X.; Zhou, Z.; Xia, Q.; Kaplan, M.J.; Kong, X.; et al. Hepatocytes and Neutrophils Cooperatively Suppress Bacterial Infection by Differentially Regulating Lipocalin-2 and Neutrophil Extracellular Traps. Hepatology 2018, 68, 1604–1620. [Google Scholar] [CrossRef]
- Jung, M.; Brüne, B.; Hotter, G.; Sola, A. Macrophage-Derived Lipocalin-2 Contributes to Ischemic Resistance Mechanisms by Protecting from Renal Injury. Sci. Rep. 2016, 6, 21950. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Feng, D.; Wu, H.; Wang, H.; Chan, Y.; Kolls, J.; Borregaard, N.; Porse, B.; Berger, T.; Mak, T.W.; et al. Liver is the Major Source of Elevated Serum Lipocalin-2 Levels after Bacterial Infection or Partial Hepatectomy: A Critical Role for IL-6/STAT3. Hepatology 2015, 61, 692–702. [Google Scholar] [CrossRef]
- Srinivasan, G.; Aitken, J.D.; Zhang, B.; Carvalho, F.A.; Chassaing, B.; Shashidharamurthy, R.; Borregaard, N.; Jones, D.P.; Gewirtz, A.T.; Vijay-Kumar, M. Lipocalin 2 Deficiency Dysregulates Iron Homeostasis and Exacerbates Endotoxin-Induced Sepsis. J. Immunol. 2012, 189, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Chen, Y.; Wu, H.; Zhang, S.; Chen, X. Prediction Value of Serum Ngal in the Diagnosis and Prognosis of Experimental Acute and Chronic Kidney Injuries. Biomolecules 2020, 10, 981. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platt, E.; Robertson, F.; Al-Rashed, A.; Klootwijk, R.; Hall, A.; Quaglia, A.; Salama, A.; Heptinstall, L.; Davidson, B. NGAL in the Development of Acute Kidney Injury in a Murine Model of Remote Ischaemic Preconditioning and Liver Ischaemia Reperfusion. Int. J. Mol. Sci. 2024, 25, 5061. https://doi.org/10.3390/ijms25105061
Platt E, Robertson F, Al-Rashed A, Klootwijk R, Hall A, Quaglia A, Salama A, Heptinstall L, Davidson B. NGAL in the Development of Acute Kidney Injury in a Murine Model of Remote Ischaemic Preconditioning and Liver Ischaemia Reperfusion. International Journal of Molecular Sciences. 2024; 25(10):5061. https://doi.org/10.3390/ijms25105061
Chicago/Turabian StylePlatt, Esther, Francis Robertson, Ali Al-Rashed, Riko Klootwijk, Andrew Hall, Alberto Quaglia, Alan Salama, Lauren Heptinstall, and Brian Davidson. 2024. "NGAL in the Development of Acute Kidney Injury in a Murine Model of Remote Ischaemic Preconditioning and Liver Ischaemia Reperfusion" International Journal of Molecular Sciences 25, no. 10: 5061. https://doi.org/10.3390/ijms25105061





