Comparative Transcriptome Analysis Reveals the Protective Role of Melatonin during Salt Stress by Regulating the Photosynthesis and Ascorbic Acid Metabolism Pathways in Brassica campestris
Abstract
:1. Introduction
2. Results
2.1. Exogenous Melatonin (MT) Application Reduced Electrolyte Leakage and Protected the Photosynthetic Pigments during Salt Stress
2.2. Exogenous MT Protected the Chloroplast Structure of B. campestris during Salt Stress
2.3. Exogenous MT Application Enhanced the Ascorbic Acid Content and APX Activity and Reduced the ROS Level during Salt Stress
2.4. RNA-Seq and DEG Identification
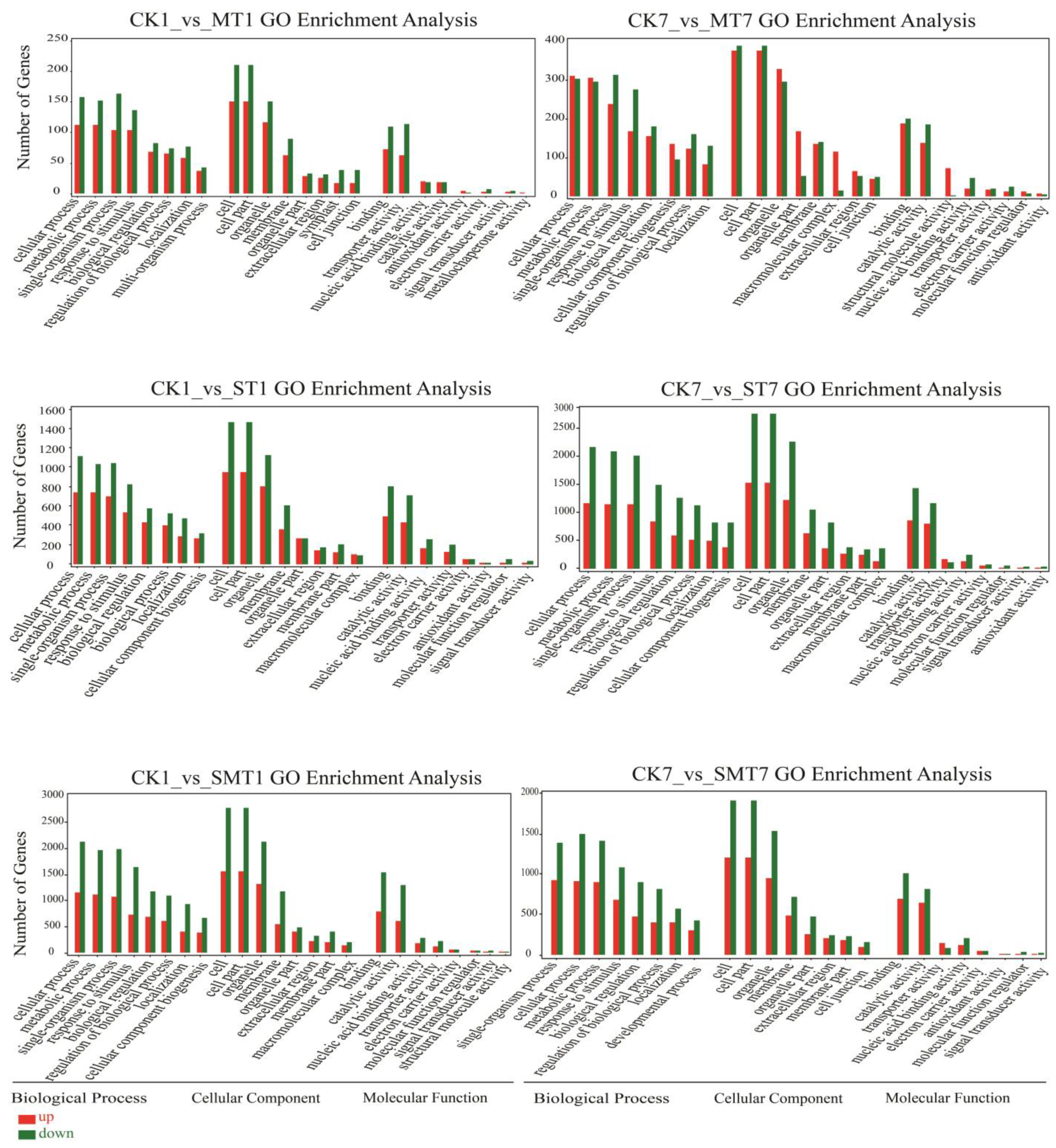
2.5. Gene Ontology Enrichment Analysis of the DEGs
2.6. KEGG Enrichment Analysis of DEGs
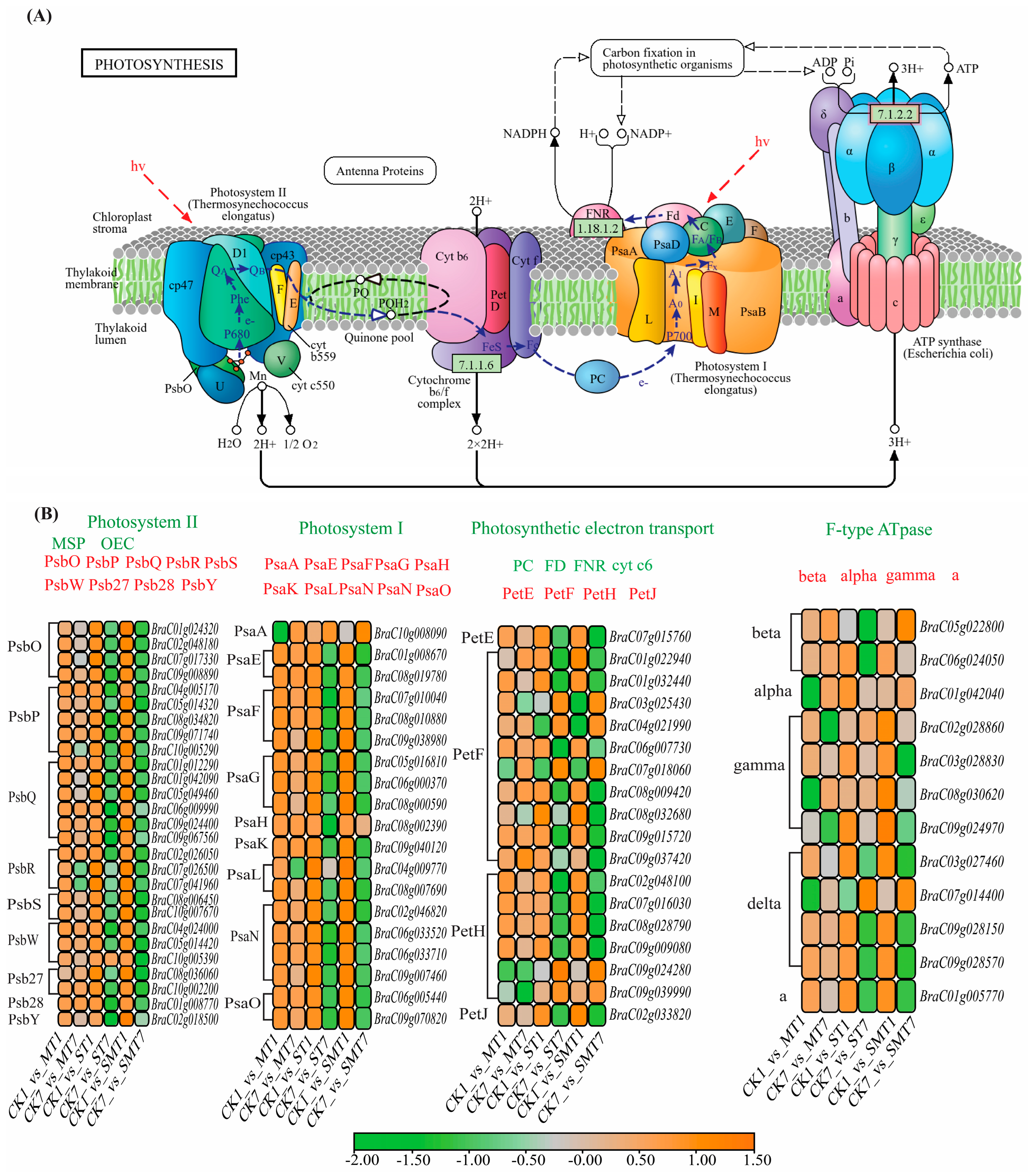
2.7. Photosynthesis-Related Gene Network Analysis of B. campestris Leaves after MT Application during Salt Stress
2.8. Ascorbic Acid Metabolism Network in B. campestris Leaves after MT Application during Salt Stress
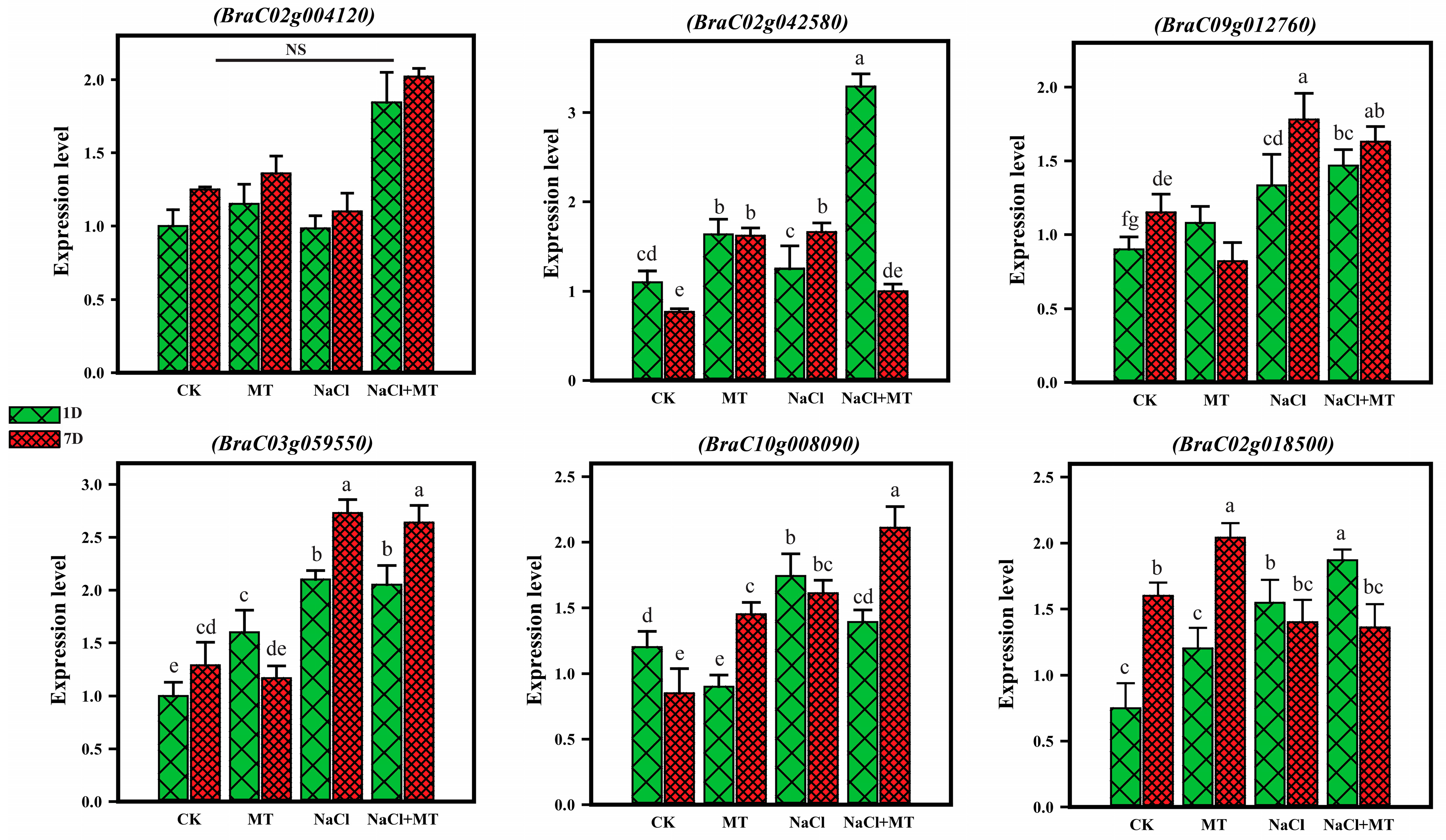
2.9. Quantitative Real-Time PCR Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Determination of Electrolyte Leakage and Chlorophyll Contents and Transmission Electron Microscopy
4.3. Determination of H2O2, DAB Staining, Ascorbic Acid Contents, and APX Activity
4.4. RNA Extraction and cDNA Library Preparation
4.5. Quantitative Real-Time PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Ahmad, N.; Zhao, S.; Zhao, C.; Zhong, W.; Wang, X.; Li, G. Effect of Salt Stress on Growth and Physiological Properties of Asparagus Seedlings. Plants 2022, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ahmad, N.; Zhang, Q.; Wakeel Umar, A.; Wang, N.; Zhao, X.; Zhou, K.; Yao, N.; Liu, X. Safflower Flavonoid 3′5′Hydroxylase Promotes Methyl Jasmonate-Induced Anthocyanin Accumulation in Transgenic Plants. Molecules 2023, 28, 3205. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, J.; Zhang, X.; Ahmad, N.; Hou, L.; Zhao, C.; Pan, J.; Tian, R.; Wang, X.; Zhao, S. The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut. Int. J. Mol. Sci. 2022, 23, 6376. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Anwar ul Haq, M.; ul Haq, T.; Ali, A.; Hussain, S.; Ibrahim, M. Protective Effect of Potassium Application on NaCl Induced Stress in Tomato (Lycopersicon esculentum L.) Genotypes. J. Plant Nutr. 2020, 43, 1988–1998. [Google Scholar] [CrossRef]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020; Volume 3, pp. 5–16. [Google Scholar]
- Jiang, D.X.; Chu, X.; Li, M.; Hou, J.J.; Tong, X.; Gao, Z.P.; Chen, G.X. Exogenous Spermidine Enhances Salt-Stressed Rice Photosynthetic Performance by Stabilizing Structure and Function of Chloroplast and Thylakoid Membranes. Photosynthetica 2020, 58, 61–71. [Google Scholar] [CrossRef]
- An, J.; Wei, X.; Huo, H. Transcriptome Analysis Reveals the Accelerated Expression of Genes Related to Photosynthesis and Chlorophyll Biosynthesis Contribution to Shade-Tolerant in Phoebe bournei. BMC Plant Biol. 2022, 22, 268. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A. Effect of Low Temperature Stress on Photosynthesis and Allied Traits: A Review. In Physiological Processes in Plants under Low Temperature Stress; Springer: Berlin/Heidelberg, Germany, 2022; pp. 199–297. [Google Scholar]
- Brini, F.; Saibi, W. Role of Proline in Regulating Physiological and Molecular Aspects of Plants under Abiotic Stress. In The Role of Growth Regulators and Phytohormones in Overcoming Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2023; pp. 317–326. [Google Scholar]
- Wang, D.; Yang, T.; Liu, R.; Li, N.; Ahmad, N.; Li, G.; Ji, Y.; Wang, C.; Li, M.; Yan, X.; et al. Large-Scale Heat-Tolerance Screening and Genetic Diversity of Pea (Pisum sativum L.) Germplasms. Plants 2022, 11, 2473. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a Fluorescence Study Revealing Effects of High Salt Stress on Photosystem II in Wheat Leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A. Prompt Chlorophyll Fluorescence as a Tool for Crop Phenotyping: An Example of Barley Landraces Exposed to Various Abiotic Stress Factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Alharbi, B.M.; Elhakem, A.H.; Alnusairi, G.S.H.; Soliman, M.H.; Hakeem, K.R.; Hasan, M.M.; Abdelhamid, M.T. Exogenous Application of Melatonin Alleviates Salt Stress-Induced Decline in Growth and Photosynthesis in Glycine max (L.) Seedlings by Improving Mineral Uptake, Antioxidant and Glyoxalase System. Plant Soil Environ. 2021, 67, 208–220. [Google Scholar] [CrossRef]
- Golding, C.; Lee, H. Study and Synthesis of Melatonin as a Strong Antioxidant in Plants. Biol. Mol. Chem. 2023, 1, 35–44. [Google Scholar]
- Ahmad, N.; Naeem, M.; Ali, H.; Alabbosh, K.F.; Hussain, H.; Khan, I.; Siddiqui, S.A.; Khan, A.A.; Iqbal, B. From Challenges to Solutions: The Impact of Melatonin on Abiotic Stress Synergies in Horticultural Plants via Redox Regulation and Epigenetic Signaling. Sci. Hortic. 2023, 321, 112369. [Google Scholar] [CrossRef]
- Li, D.; Batchelor, W.D.; Zhang, D.; Miao, H.; Li, H.; Song, S.; Li, R. Analysis of Melatonin Regulation of Germination and Antioxidant Metabolism in Different Wheat Cultivars under Polyethylene Glycol Stress. PLoS ONE 2020, 15, e0237536. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and Prospects of Melatonin in Plant Growth, Yield, and Quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef] [PubMed]
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Mangal, V.; Altaf, M.A.; Sharma, S.; Singh, B.; Kumar, M. Insight into Melatonin-Mediated Response and Signaling in the Regulation of Plant Defense under Biotic Stress. Plant Mol. Biol. 2022, 109, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin Alleviates Iron Stress by Improving Iron Homeostasis, Antioxidant Defense and Secondary Metabolism in Cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating Effects of Exogenous Melatonin on Salt Stress in Cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.; Reiter, R.J.; Wei, W.; Wang, B. Exogenously Applied Melatonin Stimulates Root Growth and Raises Endogenous Indoleacetic Acid in Roots of Etiolated Seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting Roles of Melatonin in Adventitious Root Development of Solanum Lycopersicum L. by Regulating Auxin and Nitric Oxide Signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A Small Molecule but Important for Salt Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Bai, A.; Zhao, T.; Li, Y.; Zhang, F.; Wang, H.; Shah, S.H.A.; Gong, L.; Liu, T.; Wang, Y.; Hou, X.; et al. QTL Mapping and Candidate Gene Analysis Reveal Two Major Loci Regulating Green Leaf Color in Non-Heading Chinese Cabbage. Theor. Appl. Genet. 2024, 137, 105. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Yu, Z.; Gao, Z.; Ding, Q.; Shah, S.H.A.; Lin, W.; Li, Y.; Hou, X. BcMYB111 Responds to BcCBF2 and Induces Flavonol Biosynthesis to Enhance Tolerance under Cold Stress in Non-Heading Chinese Cabbage. Int. J. Mol. Sci. 2023, 24, 8670. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F. Plant Abiotic Stress Response and Nutrient Use Efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, X.; Li, Y.; Shah, S.H.A.; Xiao, D.; Wang, J.; Hou, X.; Liu, T.; Li, Y. ETHYLENE RESPONSE FACTOR 070 Inhibits Flowering in Pak-Choi by Indirectly Impairing BcLEAFY Expression. Plant Physiol. 2024, kiae021. [Google Scholar] [CrossRef]
- Li, Y.; Si, D.; Wang, W.; Xue, S.; Shang, W.; Chi, Z.; Li, C.; Hao, C.; Govindjee, G.; Shi, Y. Light-Driven CO2 Assimilation by Photosystem II and Its Relation to Photosynthesis. Chin. J. Catal. 2023, 44, 117–126. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Z.; Duan, Y.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Transcriptomic and Metabolomic Investigation of the Adaptation Mechanisms of Blueberries to Nitrogen Deficiency Stress. Sci. Hortic. 2023, 321, 112376. [Google Scholar] [CrossRef]
- Sekar, N.; Ramasamy, R.P. Recent Advances in Photosynthetic Energy Conversion. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 19–33. [Google Scholar] [CrossRef]
- Bai, C.; Zuo, J.; Watkins, C.B.; Wang, Q.; Liang, H.; Zheng, Y.; Liu, M.; Ji, Y. Sugar Accumulation and Fruit Quality of Tomatoes under Water Deficit Irrigation. Postharvest Biol. Technol. 2023, 195, 112112. [Google Scholar] [CrossRef]
- Raziq, A.; Zhang, K.; Sun, W.; Ahmad, N.; Zhao, H.; Raza, M.A.; Ahmed, S.; Din, A.M.U.; Zhao, S.; Pan, J.; et al. Transcriptome Profiling of MYB-Overexpressed Transgenic Lines Provides Crucial Molecular Insights into Anthocyanin and Remodel the Biosynthesis Regulatory Network in Nicotiana tabacum. Ind. Crops Prod. 2024, 213, 118374. [Google Scholar] [CrossRef]
- Zhou, D.; Li, M.; Wang, X.; Li, H.; Li, Z.; Li, Q. Effects of Exogenous Melatonin on the Growth and Physiological Characteristics of Ginkgo biloba L. under Salinity Stress Conditions. Horticulturae 2024, 10, 89. [Google Scholar] [CrossRef]
- He, M.; Mei, S.; Zhai, Y.; Geng, G.; Yu, L.; Wang, Y. Effects of Melatonin on the Growth of Sugar Beet (Beta vulgaris L.) Seedlings under Drought Stress. J. Plant Growth Regul. 2023, 42, 5116–5130. [Google Scholar] [CrossRef]
- Yi, Z.; Cui, J.; Fu, Y.; Liu, H. Effect of Different Light Intensity on Physiology, Antioxidant Capacity and Photosynthetic Characteristics on Wheat Seedlings under High CO2 Concentration in a Closed Artificial Ecosystem. Photosynth. Res. 2020, 144, 23–34. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential Effects of Low Light Intensity on Broccoli Microgreens Growth and Phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The Role of Melatonin in Salt Stress Responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Sun, L.; Huang, B.; Ding, C.; Gu, Y.; Liao, J.; Hu, C.; Zhang, Z.; Yuan, S. Exogenous Melatonin Enhances Salt Stress Tolerance in Maize Seedlings by Improving Antioxidant and Photosynthetic Capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Qadir, A.; Anwar, M.; Shakoor, A.; Hayat, F. Exogenous Melatonin Enhances Salt Stress Tolerance in Tomato Seedlings. Biol. Plant. 2020, 64, 604–615. [Google Scholar] [CrossRef]
- Yuan, L.; Zheng, Y.; Nie, L.; Zhang, L.; Wu, Y.; Zhu, S.; Hou, J.; Shan, G.L.; Liu, T.K.; Chen, G. Transcriptional Profiling Reveals Changes in Gene Regulation and Signaling Transduction Pathways during Temperature Stress in Wucai (Brassica campestris L.). BMC Genom. 2021, 22, 687. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and Chloroplasts as the Original Sites of Melatonin Synthesis: A Hypothesis Related to Melatonin’s Primary Function and Evolution in Eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous Melatonin Enhances Cold, Salt and Drought Stress Tolerance by Improving Antioxidant Defense in Tea Plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.M.; Lee, I.J. Exogenous Melatonin Induces Drought Stress Tolerance by Promoting Plant Growth and Antioxidant Defence System of Soybean Plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, H.; Wang, B.; Wu, X.; Lan, R.; Huang, X.; Chen, B.; Chen, G.; Jiang, C.; Wang, J. Exogenous Melatonin Improves the Growth of Rice Seedlings by Regulating Redox Balance and Ion Homeostasis under Salt Stress. J. Plant Growth Regul. 2022, 41, 2108–2121. [Google Scholar] [CrossRef]
- Wang, F.; Wan, C.; Wu, W.; Yang, S.; Chen, X. Exogenous Melatonin (MT) Enhances Salt Tolerance of Okra (Abelmoschus esculentus L.) Plants by Regulating Proline, Photosynthesis, Ion Homeostasis and ROS Pathways. Vegetos 2024, 37, 224–238. [Google Scholar] [CrossRef]
- Celi, G.E.A.; Gratão, P.L.; Lanza, M.G.D.B.; Dos Reis, A.R. Physiological and Biochemical Roles of Ascorbic Acid on Mitigation of Abiotic Stresses in Plants. Plant Physiol. Biochem. 2023, 202, 107970. [Google Scholar] [CrossRef]
- Shigeoka, S.; Nakano, Y.; Kitaoka, S. The Biosynthetic Pathway of L-Ascorbic Acid in Euglena gracilis Z. J. Nutr. Sci. Vitaminol. 1979, 25, 299–307. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shigeoka, S. Recent Advances in Ascorbate Biosynthesis and the Physiological Significance of Ascorbate Peroxidase in Photosynthesizing Organisms. Biosci. Biotechnol. Biochem. 2008, 72, 1143–1154. [Google Scholar] [CrossRef]
- Foyer, C.H. Redox Homeostasis: Opening up Ascorbate Transport. Nat. Plants 2015, 1, 14012. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Al Mahmud, J.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Liu, J.; Ahmad, N.; Hong, Y.; Zhu, M.; Zaman, S.; Wang, N.; Yao, N.; Liu, X. Molecular Characterization of an Isoflavone 2′-Hydroxylase Gene Revealed Positive Insights into Flavonoid Accumulation and Abiotic Stress Tolerance in Safflower. Molecules 2022, 27, 8001. [Google Scholar] [CrossRef]
- Shalata, A.; Mittova, V.; Volokita, M.; Guy, M.; Tal, M. Response of the Cultivated Tomato and Its Wild Salt-tolerant Relative Lycopersiconpennellii to Salt-dependent Oxidative Stress: The Root Antioxidative System. Physiol. Plant. 2001, 112, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kusvuran, S.; Ellialtioglu, S.; Polat, Z. Antioxidative Enzyme Activity, Lipid Peroxidation, and Proline Accumulation in the Callus Tissues of Salt and Drought Tolerant and Sensitive Pumpkin Genotypes under Chilling Stress. Hortic. Environ. Biotechnol. 2013, 54, 319–325. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Li, H.J.; Zhao, C.F.; Xue, J.Q.; Zhang, R.H. Exogenous Melatonin Improves Drought Tolerance in Maize Seedlings by Regulating Photosynthesis and the Ascorbate–Glutathione Cycle. Russ. J. Plant Physiol. 2020, 67, 809–821. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, Y.Y.; Liu, J.; Liu, H.T.; Liu, H.P.; Kang, G.Z. Exogenous Melatonin Mitigates Cadmium Toxicity through Ascorbic Acid and Glutathione Pathway in Wheat. Ecotoxicol. Environ. Saf. 2022, 237, 113533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin Increases the Chilling Tolerance of Chloroplast in Cucumber Seedlings by Regulating Photosynthetic Electron Flux and the Ascorbate-Glutathione Cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Y.; Liu, X.; Ahmad, N.; Wang, N.; Jin, L.; Yao, N.; Liu, X. A Cinnamate 4-HYDROXYLASE1 from Safflower Promotes Flavonoids Accumulation and Stimulates Antioxidant Defense System in Arabidopsis. Int. J. Mol. Sci. 2023, 24, 5393. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R. Melatonin Improves Drought Stress Tolerance of Tomato by Modulating Plant Growth, Root Architecture, Photosynthesis, and Antioxidant Defense System. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.K.; Castagna, A.; Salem, E.; Ranieri, A.; Abdelly, C. Sea Fennel (Crithmum maritimum L.) under Salinity Conditions: A Comparison of Leaf and Root Antioxidant Responses. Plant Growth Regul. 2007, 53, 185–194. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta (BBA)-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Zhang, Q.; Ahmad, N.; Li, Z.; He, J.; Wang, N.; Naeem, M.; Jin, L.; Yao, N.; Liu, X. CtCYP71A1 Promotes Drought Stress Tolerance and Lignin Accumulation in Safflower and Arabidopsis. Environ. Exp. Bot. 2023, 213, 105430. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Yuan, S.; Hu, D.; Wang, Y.; Shao, C.; Liu, T.; Zhang, C.; Cheng, F.; Hou, X.; Li, Y. BcWRKY1 Confers Salt Sensitivity via Inhibiting Reactive Oxygen Species Scavenging. Plant Mol. Biol. 2022, 109, 741–759. [Google Scholar] [CrossRef]
- Kamal, O.M.; Shah, S.H.A.; Li, Y.; Hou, X.; Li, Y. Production of Ascorbic Acid, Total Protein, Callus and Root In Vitro of Non-Heading Chinese Cabbage by Tissue Culture. Mol. Biol. Rep. 2020, 47, 6887–6897. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Ahmad, N.; Li, T.; Liu, Y.; Hoang, N.Q.V.; Ma, X.; Zhang, X.; Liu, J.; Yao, N.; Liu, X.; Li, H. Molecular and Biochemical Rhythms in Dihydroflavonol 4-Reductase- Mediated Regulation of Leucoanthocyanidin Biosynthesis in Carthamus tinctorius L. Ind. Crops Prod. 2020, 156, 112838. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.H.A.; Wang, H.; Xu, H.; Yu, Z.; Hou, X.; Li, Y. Comparative Transcriptome Analysis Reveals the Protective Role of Melatonin during Salt Stress by Regulating the Photosynthesis and Ascorbic Acid Metabolism Pathways in Brassica campestris. Int. J. Mol. Sci. 2024, 25, 5092. https://doi.org/10.3390/ijms25105092
Shah SHA, Wang H, Xu H, Yu Z, Hou X, Li Y. Comparative Transcriptome Analysis Reveals the Protective Role of Melatonin during Salt Stress by Regulating the Photosynthesis and Ascorbic Acid Metabolism Pathways in Brassica campestris. International Journal of Molecular Sciences. 2024; 25(10):5092. https://doi.org/10.3390/ijms25105092
Chicago/Turabian StyleShah, Sayyed Hamad Ahmad, Haibin Wang, Huanhuan Xu, Zhanghong Yu, Xilin Hou, and Ying Li. 2024. "Comparative Transcriptome Analysis Reveals the Protective Role of Melatonin during Salt Stress by Regulating the Photosynthesis and Ascorbic Acid Metabolism Pathways in Brassica campestris" International Journal of Molecular Sciences 25, no. 10: 5092. https://doi.org/10.3390/ijms25105092




