Microgravity-like Crystallization of Paramagnetic Species in Strong Magnetic Fields
Abstract
:1. Introduction
2. Results and Discussion
2.1. Theoretical Considerations
2.2. Magnetic Field Characterization
2.3. In-Magnet Crystal Growth Procedure
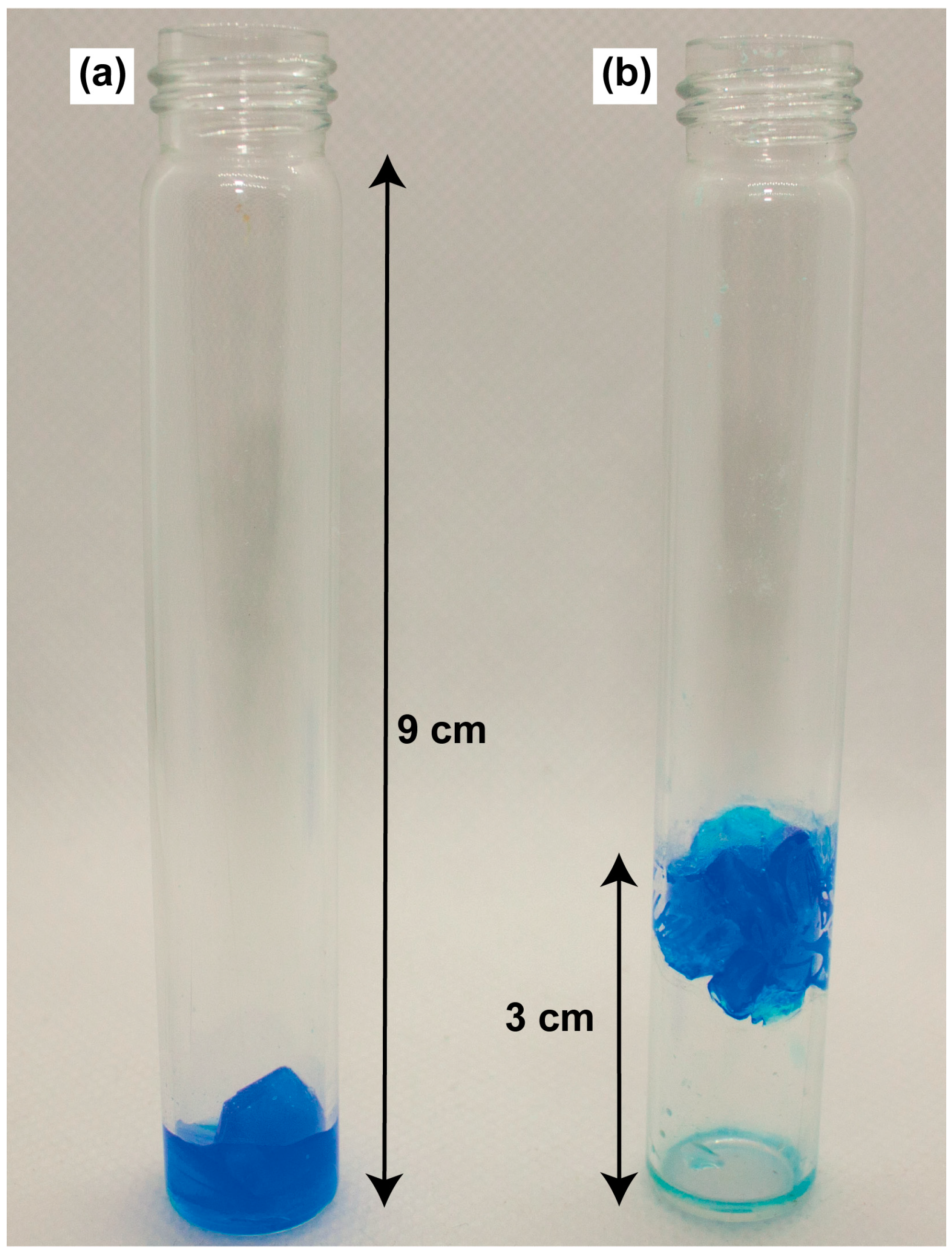
2.4. Growth of CuSO4∙5H2O Crystals
2.5. Growth from a Solution of a Mixture of Copper and Cobalt Sulfates
- (1)
- When the magnetic field gradient balances out the gravitational force, magnetic dipole interactions between crystallization centers can induce crystal drift. The drift phenomenon can take place at a rate that exceeds the thermal rate and is observed in crystals with a volume exceeding (0.04 mm3), located at a distance roughly equivalent to the diameter of the test tubes utilized (1 cm). Depending on the relative position of crystals, the dipole force can lead to either repulsion or attraction of the crystals. However, attraction occurs more frequently than repulsion, causing the crystal centers to stick together and form a single center. Considering that the final size of the developed crystals significantly exceeds 0.1 mm, it is likely that this attraction and subsequent merging due to dipole forces plays a substantial role in the observed crystal growth patterns (detailed analysis is provided in Section G1 of the Supplementary Materials).
- (2)
- When crystals are situated in the magnetic field of an NMR magnet, they are subjected to both axial force and radial forces. Assuming that the test tube in practical experiments has some off-center displacement from the axis of the magnet, it results in force acting along the perimeter of the tube. Consequently, this creates a “pocket” where the crystals are collected and aggregate, potentially influencing the overall crystal growth (refer to Section G2 of the Supplementary Materials for more details).
- (3)
- During crystal growth, a zone depleted of dissolved species forms around the crystal, leading to a slight reduction in the solution’s density in this vicinity. In the absence of a magnetic field, this density gradient leads to the initiation of natural convection, similar to the convection observed in a heated liquid. In the presence of a magnetic field, the convection can be significantly suppressed due to the appearance of a force that compensates for gravitational force. This phenomenon has been partially studied and is supported by evidence in the literature [11,12,13,32]. The suppression of convection near the growing crystal means it grows in a solution with a lower local concentration compared to the bulk solution. This change in the local concentration impacts the rate of the nucleation process. With the reduced concentration, the likelihood of new crystal nucleation decreases, leading to a slower nucleation rate [33]. As a result, rather than the formation of numerous small crystals in proximity of a seed, the growth conditions favor the development of a single large crystal. Thus, the suppression of convection can play a crucial role in promoting the growth of a predominant crystal, significantly affecting the final size of the crystal (for additional details, refer to Section G3 of the Supplementary Materials).
3. Materials and Methods
3.1. Superconducting Magnet
3.2. Experimental Setup
3.3. Analysis Methods
3.3.1. UV–Visible Spectrophotometry
3.3.2. Single-Crystal X-ray Diffraction
3.3.3. Elemental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McPherson, A.; DeLucas, L.J. Microgravity protein crystallization. Npj Microgravity 2015, 1, 15010. [Google Scholar] [CrossRef]
- Drago, V.N.; Devos, J.M.; Blakeley, M.P.; Forsyth, V.T.; Kovalevsky, A.Y.; Schall, C.A.; Mueser, T.C. Microgravity crystallization of perdeuterated tryptophan synthase for neutron diffraction. Npj Microgravity 2022, 8, 13. [Google Scholar] [CrossRef]
- Ahari, H.; Bedard, R.L.; Bowes, C.L.; Coombs, N.; Dag, O.; Jiang, T.; Ozin, G.A.; Petrov, S.; Sokolov, I.; Verma, A.; et al. Effect of microgravity on the crystallization of a self-assembling layered material. Nature 1997, 388, 857–860. [Google Scholar] [CrossRef]
- Simon, M.D.; Geim, A.K. Diamagnetic levitation: Flying frogs and floating magnets (invited). J. Appl. Phys. 2002, 87, 6200–6204. [Google Scholar] [CrossRef]
- Palagummi, S.; Yuan, F.-G. Magnetic Levitation and Its Application for Low Frequency Vibration Energy Harvesting. In Structural Health Monitoring (SHM) in Aerospace Structures; Elsevier: Amsterdam, The Netherlands, 2016; pp. 213–251. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tanimoto, Y. (Eds.) Magneto-Science; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2006; ISBN 9780387467658. [Google Scholar]
- Landau, L.D.; Lifshitz, E.M. Electrodynamics of Continuous Media; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar] [CrossRef]
- Monzon, L.M.; Coey, J. Magnetic fields in electrochemistry: The Kelvin force. A mini-review. Electrochem. Commun. 2014, 42, 42–45. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Rhen, F.M.F.; Dunne, P.; McMurry, S. The magnetic concentration gradient force—Is it real? J. Solid State Electrochem. 2007, 11, 711–717. [Google Scholar] [CrossRef]
- Quettier, L.; Vincent-Viry, O.; Mailfert, A.; Juster, F.P. Micro-gravity: Superconducting coils for crystal growth. Influence of the levitation force on natural convection in the fluid. Eur. Phys. J. Appl. Phys. 2003, 22, 69–73. [Google Scholar] [CrossRef]
- Poodt, P.W.G.; Heijna, M.C.R.; Christianen, P.C.M.; van Enckevort, W.J.P.; de Grip, W.J.; Tsukamoto, K.; Maan, J.C.; Vlieg, E. Using Gradient Magnetic Fields to Suppress Convection during Crystal Growth. Cryst. Growth Des. 2006, 6, 2275–2280. [Google Scholar] [CrossRef]
- Poodt, P.W.G.; Heijna, M.C.R.; Christianen, P.C.M.; van Enckevort, W.J.P.; Maan, J.C.; Vlieg, E. A Comparison between Simulations and Experiments for Microgravity Crystal Growth in Gradient Magnetic Fields. Cryst. Growth Des. 2008, 8, 2200–2204. [Google Scholar] [CrossRef]
- Poodt, P.W.G.; Heijna, M.C.R.; Tsukamoto, K.; de Grip, W.J.; Christianen, P.C.M.; Maan, J.C.; van Enckevort, W.J.P.; Vlieg, E. Suppression of convection using gradient magnetic fields during crystal growth of NiSO4∙6H2O. Appl. Phys. Lett. 2005, 87, 214105. [Google Scholar] [CrossRef]
- Braithwaite, D.; Beaugnon, E.; Tournier, R. Magnetically controlled convection in a paramagnetic fluid. Nature 1991, 354, 134–136. [Google Scholar] [CrossRef]
- Li, L.; Suo, Y.; Liu, T.; Wang, X.; Esling, C.; Cui, J. Effect of a High Magnetic Field on Crystal Growth in the Solidification of Hypoperitectic Zn–Ag Alloy. Cryst. Growth Des. 2019, 19, 6448–6462. [Google Scholar] [CrossRef]
- Potticary, J.; Hall, C.L.; Guo, R.; Price, S.L.; Hall, S.R. On the Application of Strong Magnetic Fields during Organic Crystal Growth. Cryst. Growth Des. 2021, 21, 6254–6265. [Google Scholar] [CrossRef]
- Wakayama, N.I. Effects of a Strong Magnetic Field on Protein Crystal Growth. Cryst. Growth Des. 2002, 3, 17–24. [Google Scholar] [CrossRef]
- Surade, S.; Ochi, T.; Nietlispach, D.; Chirgadze, D.; Moreno, A. Investigations into Protein Crystallization in the Presence of a Strong Magnetic Field. Cryst. Growth Des. 2009, 10, 691–699. [Google Scholar] [CrossRef]
- Yang, X.; Tschulik, K.; Uhlemann, M.; Odenbach, S.; Eckert, K. Magnetic Separation of Paramagnetic Ions From Initially Homogeneous Solutions. IEEE Trans. Magn. 2014, 50, 4600804. [Google Scholar] [CrossRef]
- Okada, S.; Mishima, F.; Akiyama, Y.; Nishijima, S. Fundamental study on recovery of resources by magnetic separation using superconducting bulk magnet. Phys. C Supercond. Its Appl. 2011, 471, 1520–1524. [Google Scholar] [CrossRef]
- A Butcher, T.; Coey, J.M.D. Magnetic forces in paramagnetic fluids. J. Phys. Condens. Matter 2022, 35, 053002. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.-C.; Geng, L.-Q.; Lu, Q.-Q.; Lu, H.-M.; Shang, P.; Wakayama, N.I. Multiple Orientation Responses of Lysozyme Crystals to Magnetic Field When Paramagnetic Salts Are Used As the Crystallization Agents. Cryst. Growth Des. 2009, 9, 5083–5091. [Google Scholar] [CrossRef]
- Maki, S.; Oda, Y.; Ataka, M. High-quality crystallization of lysozyme by magneto-Archimedes levitation in a superconducting magnet. J. Cryst. Growth 2004, 261, 557–565. [Google Scholar] [CrossRef]
- Schieber, M. The effects of high magnetic fields on the isothermal dissolution and growth rates of Fe(NH4)2(SO4)2·6H2O and KAl(SO4)2·12H2O seed crystals. J. Cryst. Growth 1967, 1, 131–138. [Google Scholar] [CrossRef]
- Kuschel, F.; König, A.; Gropp, R. Crystal Growth in Magnetic Fields (I) Crystallization of Me(NH4)2(SO4)2·6H2O (Me = Zn, Cu, Ni, Fe) from Aqueous Solutions in Moderate Magnetic Fields. Cryst. Res. Technol. 1982, 17, 793–799. [Google Scholar] [CrossRef]
- Kuschel, F.; König, A. Crystal Growth in Magnetic Fields (II) Crystallization of Co(NH4)2(SO4)2·6H2O from Aqueous Solutions in High Magnetic Fields. Cryst. Res. Technol. 1982, 17, 801–806. [Google Scholar] [CrossRef]
- Freitas, A.M.B.; Landgraf, F.J.G.; Nvltý, J.; Giulietti, M. Influence of Magnetic Field in the Kinetics of Crystallization of Diamagnetic and Paramagnetic Inorganic Salts. Cryst. Res. Technol. 1999, 34, 1239–1244. [Google Scholar] [CrossRef]
- Hirosawa, S.; Nishino, M.; Miyashita, S. Perspectives for high-performance permanent magnets: Applications, coercivity, and new materials. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013002. [Google Scholar] [CrossRef]
- Uglietti, D. A review of commercial high temperature superconducting materials for large magnets: From wires and tapes to cables and conductors. Supercond. Sci. Technol. 2019, 32, 053001. [Google Scholar] [CrossRef]
- Gan, Z.; Kwak, H.-T.; Bird, M.; Cross, T.; Gor’kov, P.; Brey, W.; Shetty, K. High-field NMR using resistive and hybrid magnets. J. Magn. Reson. 2008, 191, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Redhammer, G.J.; Koll, L.; Bernroider, M.; Tippelt, G.; Amthauer, G.; Roth, G. Co2+-Cu2+ substitution in bieberite solid-solution series, (Co1−xCux)SO4·7H2O, 0.00 ≤ x ≤ 0.46: Synthesis, single-crystal structure analysis, and optical spectroscopy. Am. Miner. 2007, 92, 532–545. [Google Scholar] [CrossRef]
- Poodt, P.W.G.; Christianen, P.C.M.; van Enckevort, W.J.P.; Maan, J.C.; Vlieg, E. The Critical Rayleigh Number in Low Gravity Crystal Growth from Solution. Cryst. Growth Des. 2008, 8, 2194–2199. [Google Scholar] [CrossRef]
- Girshick, S.L. The dependence of homogeneous nucleation rate on supersaturation. J. Chem. Phys. 2014, 141, 024307. [Google Scholar] [CrossRef]
- Bruker, A. Saint-Plus and Xprep; Bruker Axs Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K. Inequalities for the Modified Bessel Function of First Kind of Non-Negative Order. Math. Anal. Appl. 2023, 524, 127082. [Google Scholar] [CrossRef]
- Ravaud, R.; Lemarquand, G. Discussion about the Magnetic Field Produced by Cylindrical Halbach Structures. Prog. Electromagn. Res. B 2009, 13, 275–308. [Google Scholar] [CrossRef]
- Doğan, N.; Topkaya, R.; Subaşi, H.; Yerli, Y.; Rameev, B. Development of Halbach Magnet for Portable NMR Device. J. Phys. Conf. Ser. 2009, 153, 012047. [Google Scholar] [CrossRef]
- Tolstikov, S.E.; Artiukhova, N.A.; Romanenko, G.V.; Bogomyakov, A.S.; Zueva, E.M.; Barskaya, I.Y.; Fedin, M.V.; Maryunina, K.Y.; Tretyakov, E.V.; Sagdeev, R.Z.; et al. Heterospin Complex Showing Spin Transition at Room Temperature. Polyhedron 2015, 100, 132–138. [Google Scholar] [CrossRef]
- Wilcox, W.R. Transport Phenomena in Crystal Growth from Solution. Prog. Cryst. Growth Charact. Mater. 1993, 26, 153–194. [Google Scholar] [CrossRef]




| x (Content of Cu in CuxCo1−xSO4∙7H2O) | ~0.4 | ~0.4 | 0.46 [31] |
|---|---|---|---|
| Magnetic field, T | ~7 | Ambient conditions | Ambient conditions |
| M1–O1 | 2.0187(12) | 2.0229(16) | 2.017(1) |
| M1–O2 | 2.0965(13) | 2.0988(18) | 2.099(1) |
| M1–O3 | 2.1256(12) | 2.1206(16) | 2.124(1) |
| M2–O4 | 1.9927(11) | 1.9859(14) | 2.001(1) |
| M2–O5 | 1.9972(11) | 1.9894(16) | 2.003(1) |
| M2–O6 | 2.3107(13) | 2.3259(18) | 2.296(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsonenko, A.A.; Artiukhova, N.A.; Letyagin, G.A.; Kiryutin, A.S.; Zhukov, I.V.; Veber, S.L. Microgravity-like Crystallization of Paramagnetic Species in Strong Magnetic Fields. Int. J. Mol. Sci. 2024, 25, 5110. https://doi.org/10.3390/ijms25105110
Samsonenko AA, Artiukhova NA, Letyagin GA, Kiryutin AS, Zhukov IV, Veber SL. Microgravity-like Crystallization of Paramagnetic Species in Strong Magnetic Fields. International Journal of Molecular Sciences. 2024; 25(10):5110. https://doi.org/10.3390/ijms25105110
Chicago/Turabian StyleSamsonenko, Arkady A., Natalia A. Artiukhova, Gleb A. Letyagin, Alexey S. Kiryutin, Ivan V. Zhukov, and Sergey L. Veber. 2024. "Microgravity-like Crystallization of Paramagnetic Species in Strong Magnetic Fields" International Journal of Molecular Sciences 25, no. 10: 5110. https://doi.org/10.3390/ijms25105110






