Interleukin-13 Treatment of Living Lung Tissue Model Alters the Metabolome and Proteome—A Nano-DESI MS Metabolomics and Shotgun Proteomics Study
Abstract
1. Introduction
2. Results
2.1. Air–Liquid Interface Nano-DESI MS for Direct Analysis of Living Cells
2.2. Transportation and Metabolism of Histamine and Corticosteroids
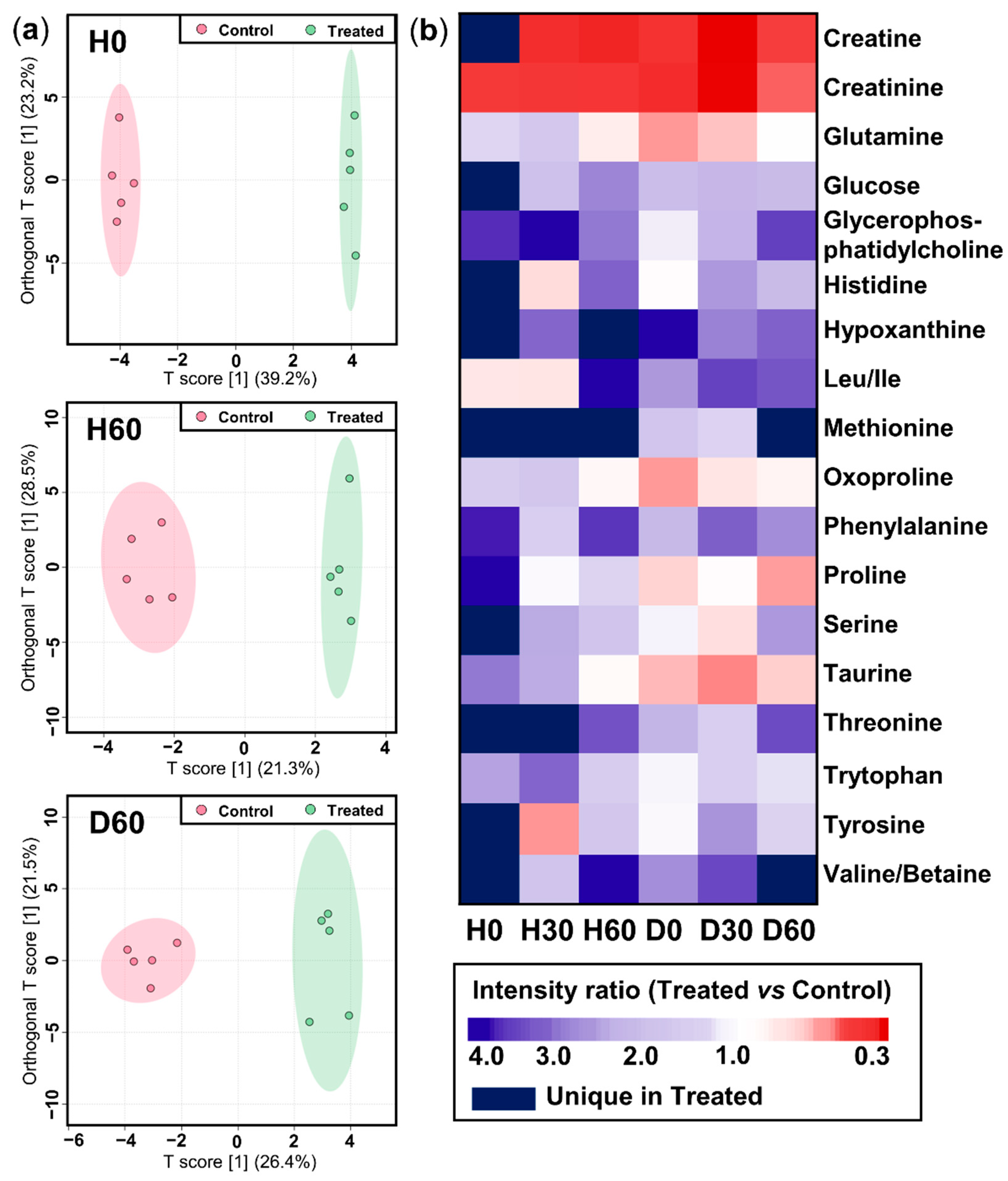
2.3. Metabolome Alterations in Asthma and Drug Treatment
2.3.1. Non-Targeted Overview of Endogenous Metabolism
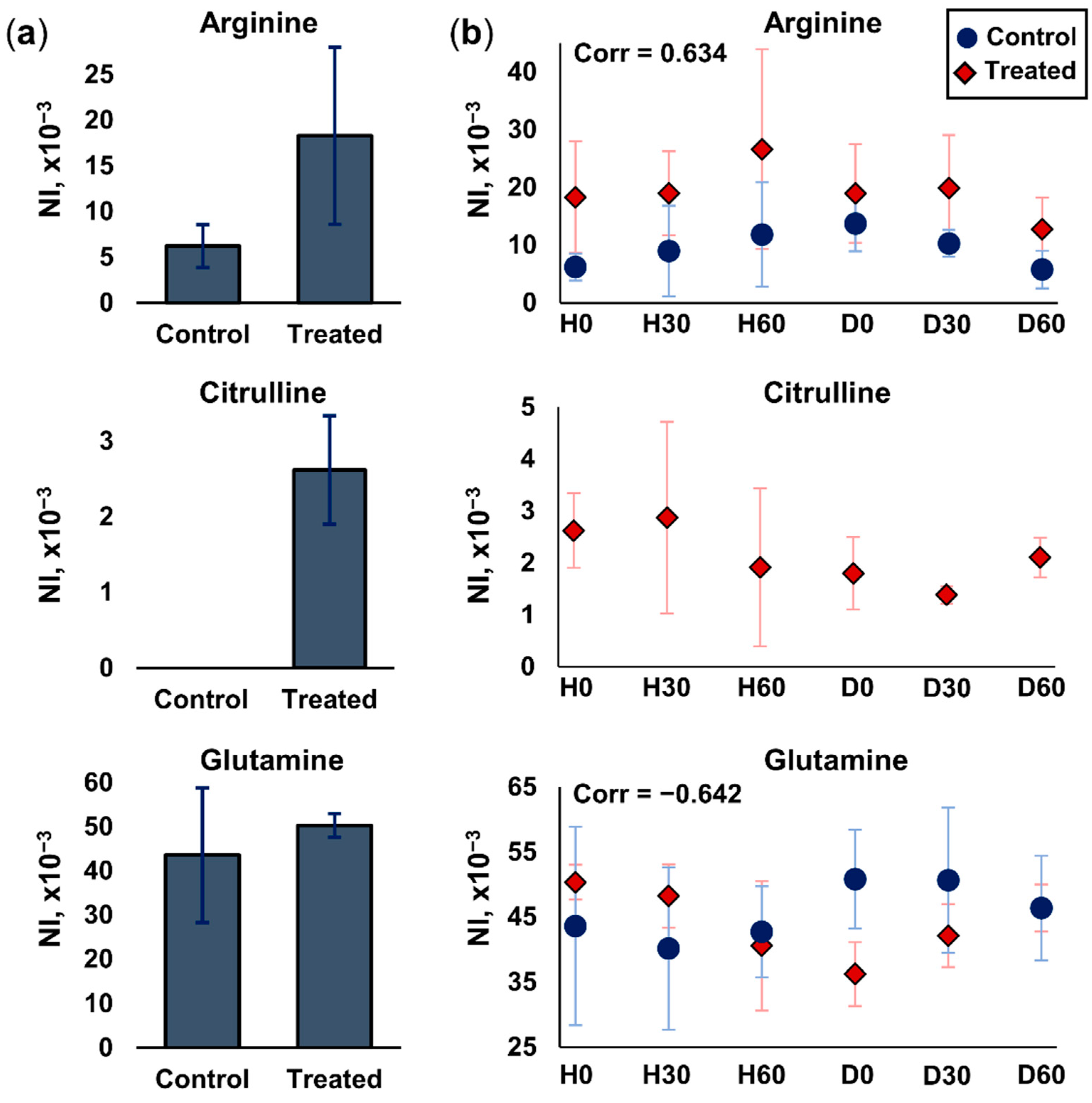
2.3.2. Arginine and Glutamine Metabolism
2.4. Proteomics Profiling after Histamine and Drug Treatment
3. Discussion
4. Materials and Methods
4.1. Reagents and Standards
4.2. Cell Culturing and Air–Liquid Interface (ALI) Model
4.3. Metabolomics Analysis
4.3.1. Instrumentation
4.3.2. Time-Resolved Treatment Investigation of ALI Cell Surface
4.4. Proteomics Analysis
4.4.1. Sample Preparation
4.4.2. NanoHPLC-MS/MS Analysis
4.5. Data Analysis
4.5.1. Metabolomics
4.5.2. Proteomics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Complex phenotypes in asthma: Current definitions. Pulm. Pharmacol. Ther. 2013, 26, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Vatrella, A.; Fabozzi, I.; Calabrese, C.; Maselli, R.; Pelaia, G. Dupilumab: A novel treatment for asthma. J. Asthma Allergy 2014, 7, 123–130. [Google Scholar] [CrossRef]
- Wills-Karp, M. Interleukin-13 in Asthma Pathogenesis. Immunol. Rev. 2004, 202, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Ogasawara, M. The Role of Histamine in the Pathophysiology of Asthma and the Clinical Efficacy of Antihistamines in Asthma Therapy. Int. J. Mol. Sci. 2019, 20, 1733. [Google Scholar] [CrossRef] [PubMed]
- Corren, J. Role of Interleukin-13 in Asthma. Curr. Allergy Asthma. Rep. 2013, 13, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Robin, L. Thurmond. Histamine in Inflammation. (2010). Available online: https://link.springer.com/book/10.1007/978-1-4419-8056-4 (accessed on 22 February 2024).
- Laitinen, L.A.; Laitinen, A.; Haahtela, T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a β2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: A randomized, double-blind, parallel-group controlled trial. J. Allergy Clin. Immunol. 1992, 90, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Alangari, A.A. Corticosteroids in the treatment of acute asthma. Ann. Thorac. Med. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hanratty, C.E.; Matthews, J.G.; Arron, J.R.; Choy, D.F.; Pavord, I.D.; Bradding, P.; Brightling, C.E.; Chaudhuri, R.; Cowan, D.C.; Djukanovic, R.; et al. A randomised pragmatic trial of corticosteroid optimization in severe asthma using a composite biomarker algorithm to adjust corticosteroid dose versus standard care: Study protocol for a randomised trial. Trials 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Li, D.; Jiang, S. Efficacy of Anti-Interleukin-5 Therapy with Mepolizumab in Patients with Asthma: A Meta-Analysis of Randomized Placebo-Controlled Trials. PLoS ONE 2013, 8, e59872. [Google Scholar]
- Clarus Leung, X.; Wadsworth, S.J.; Jasemine Yang, S.; Dorscheid, D.R. Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, 1063–1073. [Google Scholar] [CrossRef]
- Chen, S.; Schoen, J. Air-liquid interface cell culture: From airway epithelium to the female reproductive tract. Reprod. Domest. Anim. 2019, 54, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Baldassi, D.; Gabold, B.; Merkel, O.M. Air−Liquid Interface Cultures of the Healthy and Diseased Human Respiratory Tract: Promises, Challenges, and Future Directions. Adv. NanoBiomed Res. 2021, 1, 2000111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Schaefer, N.; Chu, H.W. Air–Liquid Interface Culture of Human and Mouse Airway Epithelial Cells. In Lung Innate Immunity and Inflammation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 91–109. [Google Scholar] [CrossRef]
- Ghio, A.J.; Dailey, L.A.; Soukup, J.M.; Stonehuerner, J.; Richards, J.H.; Devlin, R.B. Growth of human bronchial epithelial cells at an air-liquid interface alters the response to particle exposure. Part. Fibre Toxicol. 2013, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Everman, J.L.; Rios, C.; Seibold, M.A. Utilization of Air–Liquid Interface Cultures as an In Vitro Model to Assess Primary Airway Epithelial Cell Responses to the Type 2 Cytokine Interleukin-13. In Type 2 Immunity: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 419–432. [Google Scholar] [CrossRef]
- Schmidt, H.; Braubach, P.; Schilpp, C.; Lochbaum, R.; Neuland, K.; Thompson, K.; Jonigk, D.; Frick, M.; Dietl, P.; Wittekindt, O.H. IL-13 Impairs Tight Junctions in Airway Epithelia. Int. J. Mol. Sci. 2019, 20, 3222. [Google Scholar] [CrossRef] [PubMed]
- Seibold, M.A. Interleukin-13 Stimulation Reveals the Cellular and Functional Plasticity of the Airway Epithelium. Ann. Am. Thorac. Soc. 2018, 15, S98–S102. [Google Scholar] [CrossRef]
- Khalil, S.M.; Bernstein, I.; Kulaga, H.; Gour, N.; Rowan, N.; Lajoie, S.; Lane, A.P. Interleukin 13 (IL-13) alters hypoxia-associated genes and upregulates CD73. Int. Forum Allergy Rhinol. 2020, 10, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Moore, C.M.; Gurrola, J.G.; Pletcher, S.D.; Goldberg, A.N.; Alvarez, R.; Yamato, S.; Bratcher, P.E.; Shaughnessy, C.A.; Zeitlin, P.L.; et al. IL-13–programmed airway tuft cells produce PGE2, which promotes CFTR-dependent mucociliary function. JCI Insight 2022, 7, e159832. [Google Scholar] [CrossRef] [PubMed]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Dahlin, A.; McGeachie, M.J.; Qiu, W.; Sordillo, J.; Wan, E.S.; Wu, A.C.; Lasky-Su, J. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest 2017, 151, 262–277. [Google Scholar] [CrossRef]
- Xu, S.; Panettieri, R.A.; Jude, J. Metabolomics in asthma: A platform for discovery. Mol. Asp. Med. 2022, 85, 100990. [Google Scholar] [CrossRef]
- Aug, A.; Altraja, S.; Kilk, K.; Porosk, R.; Soomets, U.; Altraja, A. E-Cigarette Affects the Metabolome of Primary Normal Human Bronchial Epithelial Cells. PLoS ONE 2015, 10, e0142053. [Google Scholar] [CrossRef]
- López-Rodríguez, J.C.; Rodríguez-Coira, J.; Benedé, S.; Barbas, C.; Barber, D.; Villalba, M.T.; Escribese, M.M.; Villaseñor, A.; Batanero, E. Comparative metabolomics analysis of bronchial epithelium during barrier establishment after allergen exposure. Clin. Transl. Allergy 2021, 11, e12051. [Google Scholar] [CrossRef] [PubMed]
- Stollmeier, M.; Kahlert, S.; Zuschratter, W.; Oster, M.; Wimmers, K.; Isermann, B.; Rothkötter, H.-J. Air–liquid interface cultures trigger a metabolic shift in intestinal epithelial cells (IPEC-1). Histochem Cell Biol. 2023, 159, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Janocha, A.J.; Leahy, R.A.; Klatte, R.; Dudzinski, D.; Mavrakis, L.A.; Comhair, S.A.A.; Lauer, M.E.; Cotton, C.U.; Erzurum, S.C. A novel method for pulmonary research: Assessment of bioenergetic function at the air–liquid interface. Redox Biol. 2014, 2, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Meissner, F.; Geddes-McAlister, J.; Mann, M.; Bantscheff, M. The emerging role of mass spectrometry-based proteomics in drug discovery. Nat. Rev. Drug Discov. 2022, 21, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.V.; Hu, Y.-J. Integrative Analysis of Multi-omics Data for Discovery and Functional Studies of Complex Human Diseases. Adv. Genet. 2016, 93, 147–190. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Palacios, C.; Lanekoff, I. Direct Analysis of Pharmaceutical Drugs Using Nano-DESI MS. J. Anal. Methods Chem. 2016, 2016, 3591908. [Google Scholar] [CrossRef] [PubMed]
- Bergman, H.M.; Lanekoff, I. Profiling and quantifying endogenous molecules in single cells using nano-DESI MS. Analyst 2017, 142, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Bron, A.J. An Estimate of the Human Lens Epithelial Cell Size in vivo. Exp. Eye Res. 1987, 44, 899–906. [Google Scholar] [CrossRef]
- Mansur, A.H.; Afridi, L.; Sullivan, J.; Ayres, J.G.; Wilson, D. Continuous terbutaline infusion in severe asthma in adults: A retrospective study of long-term efficacy and safety. J. Asthma 2014, 51, 1076–1082. [Google Scholar] [CrossRef]
- Rimmer, C.; Hetelekides, S.; Eliseeva, S.I.; Georas, S.N.; Veazey, J.M. Budesonide promotes airway epithelial barrier integrity following double-stranded RNA challenge. PLoS ONE 2021, 16, e0260706. [Google Scholar] [CrossRef]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128, 549–556.e12. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Peramuhendige, P.; King, L.; Golding, M.; Kotian, A.; Penney, M.; Shah, S.; Manevski, N. Evaluation of in vitro models for assessment of human intestinal metabolism in drug discovery. Drug Metab. Dispos. 2020, 48, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.D.; Roberts, J.K.; Orton, C.R.; Murai, T.; Fidler, T.P.; Reilly, C.A.; Ward, R.M.; Yost, G.S. Metabolic pathways of inhaled glucocorticoids by the cyp3a enzymes. Drug Metab. Dispos. 2013, 41, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Seale, J.P. Clinical pharmacokinetics of inhaled budesonide. Clin. Pharmacokinet 2001, 40, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L. Pharmacokinetic parameters of terbutaline in healthy man. An overview. Eur. J. Respir Dis. Suppl. 1984, 134, 149–160. [Google Scholar] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.D.; Bergman, H.-M.; Lanekoff, I. A pneumatically assisted nanospray desorption electrospray ionization source for increased solvent versatility and enhanced metabolite detection from tissue. Analyst 2017, 142, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Tavelin, S.; Gråsjö, J.; Taipalensuu, J.; Ocklind, G.; Artursson, P. Applications of Epithelial Cell Culture in Studies of Drug Transport. In Epithelial Cell Culture Protocols; Wise, C., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 233–272. [Google Scholar] [CrossRef]
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef]
- Smith, J.S.; Hilibrand, A.S.; Skiba, M.A.; Dates, A.N.; Calvillo-Miranda, V.G.; Kruse, A.C. Human Histamine N-Methyltransferase Pharmacogenetics: Common Genetic Polymorphisms That Alter Activity. Available online: http://www.molpharm.org (accessed on 2 February 2024.).
- Ogasawara, M.; Yamauchi, K.; Satoh, Y.I.; Yamaji, R.; Inui, K.; Jonker, J.W.; Schinkel, A.H.; Maeyama, K. Recent advances in molecular pharmacology of the histamine systems: Organic cation transporters as a histamine transporter and histamine metabolism. J. Pharmacol. Sci. 2006, 101, 24–30. [Google Scholar] [CrossRef]
- Guo, F.H.; Comhair, S.A.; Zheng, S.; Dweik, R.A.; Eissa, N.T.; Thomassen, M.J.; Calhoun, W.; Erzurum, S.C. Molecular Mechanisms of Increased Nitric Oxide (NO) in Asthma: Evidence for Transcriptional and Post-Translational Regulation of NO Synthesis. J. Immunol. 2000, 164, 5970–5980. [Google Scholar] [CrossRef]
- Lim, S.A.M.; Jatakanon, A.; John, M.; Gilbey, T.; O’CONNOR, B.J.; Chung, K.F.; Barnes, P.J. Effect of Inhaled Budesonide on Lung Function and Airway Inflammation Assessment by Various Inflammatory Markers in Mild Asthma. Am. J. Respir Crit. Care Med. 1999, 159, 22–30. Available online: http://www.atsjournals.org (accessed on 2 February 2024.). [CrossRef]
- Henricks, P.A.J.; Nijkamp, F.P. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther. 2001, 14, 409–421. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Hensley, K.; Cole, P.; Subramaniam, R.; Aksenov, M.; Aksenova, M.; Bummer, P.M.; Haley, B.E.; Carney, J.M. Oxidatively Induced Structural Alteration of Glutamine Synthetase Assessed by Analysis of Spin Label Incorporation Kinetics: Relevance to Alzheimer’s Disease. J. Neurochem. 1997, 68, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Im, Y.N.; Chung, Y.J.; Youm, J.H.; Im, S.Y.; Han, M.K.; Lee, H.K. Glutamine deficiency shifts the asthmatic state toward neutrophilic airway inflammation. Allergy: Eur. J. Allergy Clin. Immunol. 2022, 77, 1180–1191. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Wang, C.J.; Tsai, M.H.; Lin, G. Integrated metabolic and microbial analysis reveals host–microbial interactions in IgE-mediated childhood asthma. Sci. Rep. 2021, 11, 23407. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, K.A.; Stephenson, S.T.; Mohammad, A.F.; Kim, S.O.; McCarty, N.A.; Kamaleswaran, R.; Fitzpatrick, A.M.; Chandler, J.D. Exacerbation-prone pediatric asthma is associated with arginine, lysine, and methionine pathway alterations. J. Allergy Clin. Immunol. 2023, 151, 118–127.e10. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Boushey, H.A.; Dolganov, G.M.; Barker, C.S.; Yang, Y.H.; Donnelly, S.; Ellwanger, A.; Sidhu, S.S.; Dao-Pick, T.P.; Pantoja, C.; et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc. Natl. Acad. Sci. USA 2007, 104, 15858–15863. [Google Scholar] [CrossRef]
- Boyce, J.A. The role of 15 lipoxygenase 1 in asthma comes into focus. J. Clin. Investig. 2022, 132, e155884. [Google Scholar] [CrossRef]
- Wu, J.; Lin, R.; Huang, J.; Guan, W.; Oetting, W.S.; Sriramarao, P.; Blumenthal, M.N. Functional Fcgamma Receptor Polymorphisms Are Associated with Human Allergy. PLoS ONE 2014, 9, e89196. [Google Scholar] [CrossRef]
- Dong, J.; Liao, W.; Peh, H.Y.; Chan, T.K.; Tan, W.S.D.; Li, L.; Yong, A.; Wong, W.S.F. Ribosomal protein S3 gene silencing protects against experimental allergic asthma. Br. J. Pharmacol. 2017, 174, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Kidd, C.D.A.; Thompson, P.J.; Barrett, L.; Baltic, S. Histone Modifications and Asthma. The Interface of the Epigenetic and Genetic Landscapes. Am. J. Respir Cell Mol. Biol. 2015, 54, 3–12. [Google Scholar] [CrossRef]
- Wan, F.; Lenardo, M.J. The nuclear signaling of NF-κB: Current knowledge, new insights, and future perspectives. Cell Res. 2010, 20, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kinlough, C.L.; Hughey, R.P.; Jin, M.; Inoue, H.; Etling, E.; Modena, B.D.; Kaminski, N.; Bleecker, E.R.; Meyers, D.A.; et al. Sialylation of MUC4β N-glycans by ST6GAL1 orchestrates human airway epithelial cell differentiation associated with type-2 inflammation. JCI Insight 2019, 4, e122475. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz-Scroggins, M.E.; Yuan, S.; Kerr, S.C.; Dunican, E.M.; Yu, M.; Carrington, S.D.; Fahy, J.V. Abnormalities in MUC5AC and MUC5B Protein in Airway Mucus in Asthma. Am. J. Respir Crit. Care Med. 2016, 194, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Balbisi, M.; Sugár, S.; Schlosser, G.; Szeitz, B.; Fillinger, J.; Moldvay, J.; Drahos, L.; Szász, A.M.; Tóth, G. Inter- and intratumoral proteomics and glycosaminoglycan characterization of ALK rearranged lung adenocarcinoma tissues: A pilot study. Sci. Rep. 2023, 13, 6268. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Moing, A. BioStatFlow -Statistical Analysis Workflow for ‘Omics’ Data. arXiv 2020, arXiv:2007.04599v1. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, G.; Golubova, A.; Falk, A.; Lind, S.B.; Nicholas, M.; Lanekoff, I. Interleukin-13 Treatment of Living Lung Tissue Model Alters the Metabolome and Proteome—A Nano-DESI MS Metabolomics and Shotgun Proteomics Study. Int. J. Mol. Sci. 2024, 25, 5034. https://doi.org/10.3390/ijms25095034
Tóth G, Golubova A, Falk A, Lind SB, Nicholas M, Lanekoff I. Interleukin-13 Treatment of Living Lung Tissue Model Alters the Metabolome and Proteome—A Nano-DESI MS Metabolomics and Shotgun Proteomics Study. International Journal of Molecular Sciences. 2024; 25(9):5034. https://doi.org/10.3390/ijms25095034
Chicago/Turabian StyleTóth, Gábor, Anastasia Golubova, Alexander Falk, Sara Bergström Lind, Mark Nicholas, and Ingela Lanekoff. 2024. "Interleukin-13 Treatment of Living Lung Tissue Model Alters the Metabolome and Proteome—A Nano-DESI MS Metabolomics and Shotgun Proteomics Study" International Journal of Molecular Sciences 25, no. 9: 5034. https://doi.org/10.3390/ijms25095034
APA StyleTóth, G., Golubova, A., Falk, A., Lind, S. B., Nicholas, M., & Lanekoff, I. (2024). Interleukin-13 Treatment of Living Lung Tissue Model Alters the Metabolome and Proteome—A Nano-DESI MS Metabolomics and Shotgun Proteomics Study. International Journal of Molecular Sciences, 25(9), 5034. https://doi.org/10.3390/ijms25095034





