A Description of the New Hybodont Shark Genus, Columnaodus, from the Burlington and Keokuk Limestones (Carboniferous, Mississippian, Osagean) of Illinois and Iowa, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Setting
2.1.1. General Stratigraphy and Lithology
2.1.2. Age and Depositional Setting
2.1.3. Bonebed Deposition
2.2. Material Collection and Preparation
2.2.1. Sample Locations
2.2.2. Laboratory Processing
2.2.3. Repository, Taxonomy, and Terminology
3. Results
3.1. Bonebed Lithology
3.2. Systematic Paleontology
- Class Chondrichthyes Huxley, 1880 [39]
- Subclass Elasmobranchii Bonaparte, 1838 [40]
- Infraclass Euselachii Hay, 1902 [41]
- Order Hybodontiformes Maisey, 1975 [42]
- Superfamily Hybodontoidea Owen, 1846 [43]
- Family incertae sedis
- Genus Columnaodus gen. nov.
- urn:lsid: zoobank.org:act:BB92D0D8-66EE-45CE-B5F2-8FDEE2DE9EB7
3.2.1. Diagnosis
- Columnaodus witzkei sp. nov.
- urn:lsid:zoobank.org:act:09075140-9525-4461-BFCD-F3D54A9F802E
- Species diagnosis: as for the genus.
- Type locality: Cessford Construction Company Mediapolis Quarry, Mediapolis, Des Moines County, Iowa, 41.00997, −91.05281 (Figure 2b).
3.2.2. Etymology
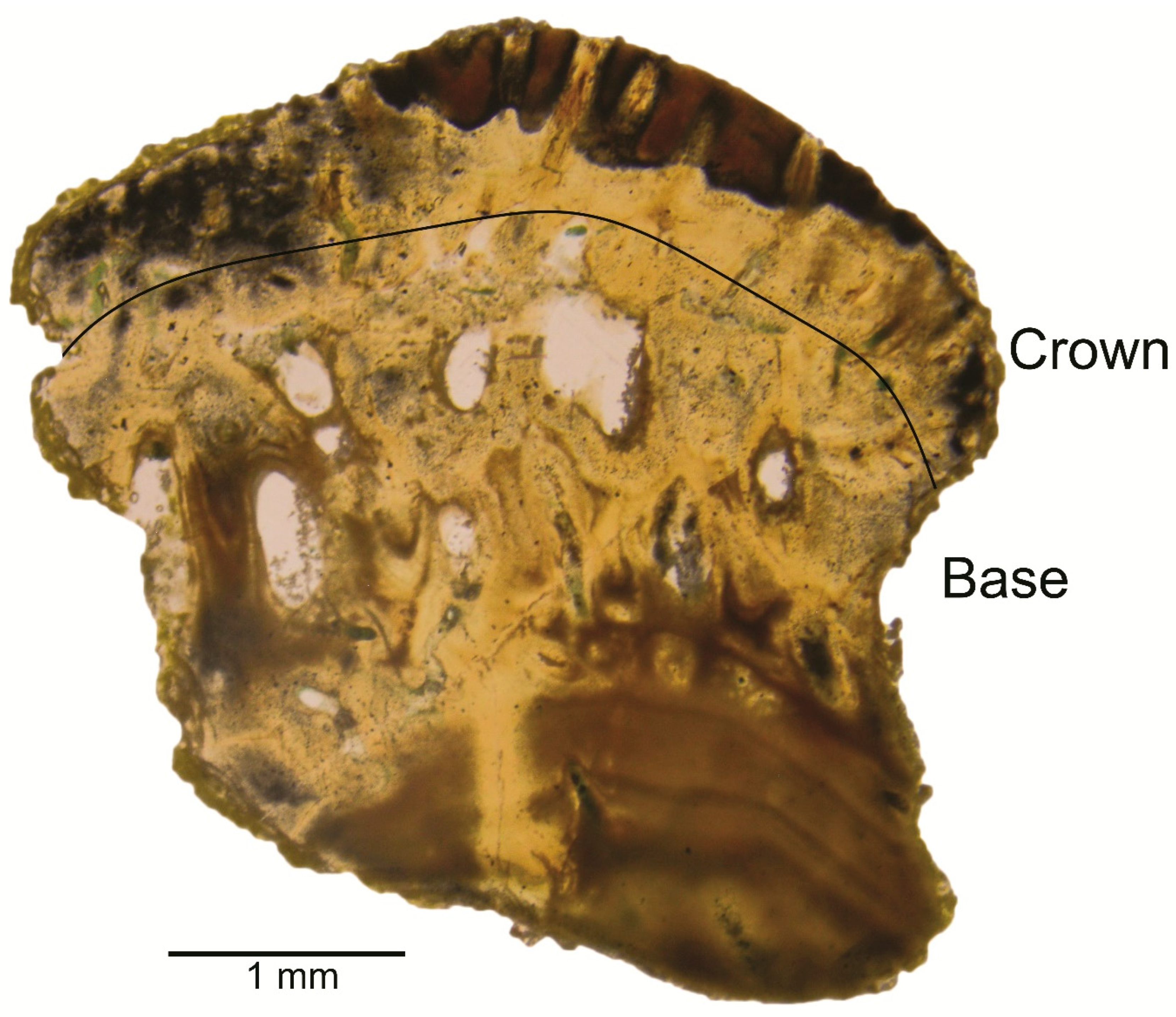
3.2.3. Description
3.2.4. Remarks
4. Discussion and Conclusions

Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witzke, B.J.; McKay, R.M.; Bunker, B.J.; Woodson, F.J. Stratigraphy and paleoenvironments of Mississippian strata in Keokuk and Washington Counties, southeast Iowa. In Guidebook Series, No. 10; Iowa Department of Natural Resources: Des Moines, IA, USA, 1990; p. 106. Available online: https://publications.iowa.gov/id/eprint/25602 (accessed on 1 April 2019).
- Newberry, J.S.; Worthen, A.H. Descriptions of new species of vertebrates, mainly from the Sub-Carboniferous limestone and coal measures of Illinois. In Geological Survey of Illinois; Worthen, A.H., Ed.; State Journal Steam Press: Springfield, IL, USA, 1866; Volume 2, pp. 9–134. [Google Scholar] [CrossRef]
- Wachsmuth, C.; Springer, F. Transition forms in crinoids, and description of five new species. Proc. Acad. Nat. Sci. Phila. 1878, 30, 224–266. Available online: https://www.jstor.org/stable/4060405 (accessed on 1 April 2019).
- Witzke, B.J.; Bunker, B.J. Relative sea-level changes during Middle Ordovician through Mississippian deposition in the Iowa area, North American Craton. In Paleozoic Sequence Stratigraphy; Views from the North American Craton; Witzke, B.J., Ludvigson, G.A., Day, J., Eds.; Geological Society of America Special Papers; Geological Society of America: McLean, VA, USA, 1996; Volume 306, pp. 307–330. [Google Scholar] [CrossRef]
- Leidy, J. Descriptions of the remains of fishes from the Carboniferous Limestone of Illinois and Missouri. Trans. Am. Philos. Soc 1860, 11, 87–90. [Google Scholar] [CrossRef]
- Newberry, J.S.; Worthen, A.H. Descriptions of fossil vertebrates. In Geological Survey of Illinois; Worthen, A.H., Ed.; State Journal Steam Press: Springfield, IL, USA, 1870; Volume 4, pp. 343–374. [Google Scholar] [CrossRef]
- St. John, O.; Worthen, A.H. Descriptions of fossil fishes. In Geological Survey of Illinois; Worthen, A.H., Ed.; State Journal Steam Press: Springfield, IL, USA, 1875; Volume 6, pp. 245–488. [Google Scholar] [CrossRef]
- Newberry, J.S. Personal letter. In Eighth, Ninth, and Tenth Annual Reports of the Geological Survey of Indiana, Made during the Years 1876-77-78; Cox, E.T., Collett, J., Levette, G.M., Eds.; Indianapolis Journal Company: Indianapolis, IN, USA, 1879; Volumes 8–10, pp. 341–349. [Google Scholar]
- St. John, O.; Worthen, A.H. Descriptions of fossil vertebrates. In Geological Survey of Illinois; Worthen, A.H., Ed.; State Journal Steam Press: Springfield, IL, USA, 1883; Volume 7, pp. 53–264. [Google Scholar] [CrossRef]
- Newberry, J.S. New species and a new genus of American Palaeozoic fishes, together with notes on the genera Oracanthus, Dactylodus, Polyrhizodus, Sandalodus, Deltodus. Trans. N. Y. Acad. Sci. 1897, 16, 282–304. [Google Scholar]
- Hay, O.P. On some changes in the names, generic and specific, of certain fossil fishes. Am. Nat. 1899, 33, 783–792. Available online: https://www.jstor.org/stable/2454274 (accessed on 1 April 2019). [CrossRef]
- Branson, E.B. Notes on some Carboniferous Cochliodonts with descriptions of seven new species. J. Geol. 1905, 13, 20–34. Available online: https://www.jstor.org/stable/30066320 (accessed on 1 April 2019). [CrossRef]
- Ginter, M.; Duffin, C.; Hampe, O. Handbook of Paleoichthyology: Chondrichthyes IV; Verlag Dr. Friedrich Pfeil: München, Germany, 2010; Volume 3D, pp. 1–168. [Google Scholar]
- Newberry, J.S. The Paleozoic fishes of North America. In Monographs of the United States Geological Survey; U.S. Government Printing Office: Washington, DC, USA, 1889; Volume 16, p. 340. [Google Scholar] [CrossRef]
- Hodnett, J.-P.M.; Elliott, D.K.; Olson, T.J. A new basal hybodont (Chondrichthyes, Hybodontiformes) from the Middle Permian (Roadian) Kaibab Formation, of northern Arizona. In The Carboniferous-Permian Transition; Lucas, S.G., DiMichele, W.A., Barrick, J.E., Schneider, J.W., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science: Albuquerque, NM, USA, 2013; Bulletin 60; pp. 103–108. [Google Scholar]
- Harris, S.E.; Parker, M.C. Stratigraphy of the Osage series in Southeastern Iowa. In Report of Investigations 1; Iowa Geological Survey: Iowa, IA, USA, 1964; pp. 1–52. [Google Scholar] [CrossRef]
- Mamet, B.L. Taxonomic note on Carboniferous Endothyracea. J. Foramin. Res. 1974, 4, 200–204. [Google Scholar] [CrossRef]
- Mamet, B.L. Foraminiferal zonation of the Lower Carboniferous: Methods and stratigraphic implications. In Concepts and Methods of Biostratigraphy; Kauffman, E.G., Hazel, J.E., Eds.; Dowden, Hutchinson, and Ross: Stroudsburg, PA, USA, 1977; pp. 445–462. [Google Scholar]
- Chauff, K.M. Multielement conodont species from the Osagean (Early Mississippian) Burlington Carbonate Shelf, Midcontinent North America, and the Chappel Limestone of Texas. Ph.D. Dissertation, University of Iowa, Iowa City, IA, USA, 1978. [Google Scholar]
- Lane, H.R. The Burlington Shelf (Mississippian, north-central United States). Geol. Palaeontol. 1978, 12, 165–176. [Google Scholar]
- Lane, H.R.; Sandberg, C.A.; Ziegler, W. Taxonomy and phylogeny of some Lower Carboniferous conodonts and preliminary standard post-Siphonodella zonation. Geol. Palaeontol. 1980, 14, 117–164. [Google Scholar]
- Chauff, K.M. Multielement conodont species and an ecological interpretation of the Lower Osagean (Lower Carboniferous) conodont zonation from Midcontinent North America. Micropaleontology 1983, 29, 404–429. Available online: https://www.jstor.org/stable/1485517 (accessed on 1 April 2019). [CrossRef]
- Lane, H.R.; Brenckle, P.L. Type Mississippian subdivisions and biostratigraphic succession. In Stratigraphy and Biostratigraphy of the Mississippian Subsystem (Carboniferous System) in Its Type Region, the Mississippi River Valley of Illinois, Missouri, and Iowa; Heckel, P.H., Ed.; Illinois State Geological Survey: Champaign, IL, USA, 2005; Guidebook 34; pp. 76–105. [Google Scholar]
- Cohen, K.M.; Finney, S.C.; Gibbard, P.L.; Fan, J.-X. The ICS International Chronostratigraphic Chart, Episodes 36. 2013. Updated. pp. 199–204. Available online: http://www.stratigraphy.org/ICSchart/ChronostratChart2023-06.pdf (accessed on 1 April 2019).
- Torsvik, T.H.; Cocks, L.R.M. Earth History and Palaeogeography; Cambridge University Press: Cambridge, UK, 2017; 317p. [Google Scholar] [CrossRef]
- Lane, H.R.; De Keyser, T.L. Paleogeography of the late Early Mississippian (Tournaisian 3) in the central and southwestern United States. In Paleozoic Paleogeography of the West-Central United States: Rocky Mountain Symposium 1; Fouch, T.D., Magathan, E.R., Eds.; Society of Economic Paleontologists and Mineralogists, Rocky Mountain Section: Fort Collins, CO, USA, 1980; pp. 149–162. [Google Scholar]
- Ross, C.A.; Ross, J.R.P. Late Paleozoic sea levels and depositional sequences. Geol. Fac. Publ. 1987, 61, 137–149. Available online: https://cedar.wwu.edu/geology_facpubs (accessed on 1 April 2019).
- Harris, D.C. Carbonate Cement Stratigraphy and Diagenesis of the Burlington Limestone (Miss.), South-East Iowa and West Illinois. Master’s Thesis, State University of New York at Stony Brook, Stony Brook, NY, USA, 1982. [Google Scholar]
- Van Tuyl, F.M. The stratigraphy of the Mississippian formations of Iowa. Iowa Geol. Surv. Annu. Rep. 1923, 30, 33–360. [Google Scholar] [CrossRef]
- Cander, H.S.; Kaufman, J.; Daniels, L.D.; Meyers, W.J. Regional dolomitization of shelf carbonates in the Burlington-Keokuk Formation (Mississippian), Illinois and Missouri: Constraints from cathodoluminescent zonal stratigraphy. In Sedimentology and Geochemistry of Dolostones; Shukla, V., Baker, P.A., Eds.; SEPM Society for Sedimentary Geology: Claremore, OK, USA, 1988; Volume 43, pp. 129–144. [Google Scholar] [CrossRef]
- Banner, J.L.; Hanson, G.N.; Meyers, W.J. Rare earth element and Nd isotopic variations in regionally extensive dolomites from the Burlington-Keokuk Formation (Mississippian): Implications for REE mobility during carbonate diagenesis. J. Sediment. Petrol. 1988, 58, 415–432. [Google Scholar] [CrossRef]
- Reif, W.E. Muschelkalk/Keuper bone-beds (Middle-Triassic, SW-Germany)-storm condensation in a regressive cycle. In Cyclic and Event Stratification; Einsele, G., Seilacher, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 299–325. [Google Scholar] [CrossRef]
- Macquaker, J.H.S. Palaeoenvironmental significance of ‘bone-beds’ in organic-rich mudstone successions: An example from the Upper Triassic of south-west Britain. Zool. J. Linn. Soc-Lond. 1994, 112, 285–308. [Google Scholar] [CrossRef]
- Brett, C.E. Sequence stratigraphy, biostratigraphy, and taphonomy in shallow marine environments. Palaios 1995, 10, 597–616. [Google Scholar] [CrossRef]
- Pyenson, N.D.; Irmis, R.B.; Lipps, J.H.; Barnes, L.G.; Mitchell, E.D., Jr.; McLeod, S.A. Origin of a widespread marine bonebed deposited during the middle Miocene Climatic Optimum. Geology 2009, 37, 519–522. [Google Scholar] [CrossRef]
- Jeppsson, L.; Fredholm, D.; Mattiasson, B. Acetic acid and phosphatic fossils: A warning. J. Paleontol. 1985, 59, 952–956. Available online: https://www.jstor.org/stable/1304939 (accessed on 4 October 2023).
- Jeppsson, L.; Anehus, R. A buffered formic acid technique for conodont extraction. J. Paleontol. 1995, 69, 790–794. Available online: https://www.jstor.org/stable/1306313 (accessed on 4 October 2023). [CrossRef]
- Jeppsson, L.; Anehus, R.; Fredholm, D. The optimal acetate buffered acetic acid technique for extracting phosphatic fossils. J. Paleontol. 1999, 73, 964–972. Available online: https://www.jstor.org/stable/1306854 (accessed on 4 October 2023). [CrossRef]
- Huxley, T. A Manual of the Anatomy of Vertebrated Animals; D. Appleton and Co.: New York, NY, USA, 1880; pp. 1–431. [Google Scholar]
- Bonaparte, C.L.J.L. Selachorum tabula analytica. Systema Icthyologicum. Mem. Soc. Neuchatel. Sci. Nat. 1838, 2, 1–16. [Google Scholar]
- Hay, O.P. Bibliography and catalogue of the fossil Vertebrata of North America. In Bulletin of the United States Geological Survey; US Government Printing Office: Washington, DC, USA, 1902; No. 179; pp. 1–868. [Google Scholar]
- Maisey, J.G. The interrelationships of phalacanthous selachians. Neues Jahr. Geol. Palaeontol. Monatsh. 1975, 9, 563–567. [Google Scholar]
- Owen, R. Lectures on the Comparative Anatomy and Physiology of the Vertebrate Animals: Delivered at the Royal College of Surgeons of England, in 1844 and 1846. Part 1 Fishes; Longman, Brown, Green, and Longmans: London, UK, 1846; pp. 1–308. [Google Scholar] [CrossRef]
- Enault, S.; Guinot, G.; Koot, M.B.; Cuny, G. Chondrichthyan tooth enameloid: Past, present, and future. Zool. J. Linn. Soc-Lond. 2015, 174, 549–570. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Duffin, C.J.; Naugolnykh, S.V. A new euselachian shark from the early Permian of the Middle Urals, Russia. Acta Palaeontol. Pol. 2017, 62, 289–298. [Google Scholar] [CrossRef]
- Kriwet, J. Late Jurassic selachians (Chondrichthyes: Hybodontiformes, Neoselachii) from southern Germany: Re-evaluation on taxonomy and diversity. Zitteliana A 2004, 44, 67–95. [Google Scholar]
- Rees, J. Interrelationships of Mesozoic hybodont sharks as indicated by dental morphology—Preliminary results. Acta Geol. Pol. 2002, 58, 217–221. [Google Scholar]
- Wen, W.; Zhang, Z.; Kriwet, J.; Hu, S.; Zhour, C.; Huang, J.; Cui, X.; Min, X.; Benton, M.J. First occurrence of hybodontid teeth in the Louping Biota (Middle Triassic, Anisian) and recovery of the marine ecosystem after the end-Permian mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 617, 111471. [Google Scholar] [CrossRef]
- Dick, R.F. On the Carboniferous shark Tristychius arcuatus Agassiz from Scotland. Earth Environ. Sci. Trans. R. Soc. 1978, 70, 63–109. [Google Scholar] [CrossRef]
- Maisch, M.W.; Matzke, A.T. A new hybodontid shark (Chondrichthyes, Hybodontiformes) from the Lower Jurassic Posidonienschiefer Formation of Dotternhause, SW Germany. Neues Jahrb. Geol. Paläontologie Abh. 2016, 280, 241–257. [Google Scholar] [CrossRef]
- Murry, P.A.; Kirby, R.E. A new hybodont shark from the Chinle and Bull Canyon Formations, Arizona, Utah, and New Mexico. In Upper Triassic Stratigraphy and Paleontology; Heckert, A.B., Lucas, S.G., Eds.; New Mexico Museum of Natural History: Albuquerque, NM, USA, 2002; Bulletin 21; pp. 87–106. [Google Scholar]
- Fischer, J.; Schneider, J.W.; Ronchi, A. New hybondontoid shark from the Permocarboniferous (Gzhelian-Asselian) of Guardia Pisano (Sardinia, Italy). Acta Palaeontol. Pol. 2010, 55, 241–264. [Google Scholar] [CrossRef]
- Wang, N.Z.; Zhang, X.; Zhu, M.; Zhao, W.J. A new articulated hybodontoid from Late Permian of northwestern China. Acta Zool. Stockholm 2009, 90, 159–170. [Google Scholar] [CrossRef]
- Bhat, M.S.; Ray, S.; Datta, P.M. A new hybodont shark (Chondrichthyes, Elasmobranchii) from the Upper Triassic Tiki Formation of India with remarks on its dental histology and biostratigraphy. J. Paleontol. 2018, 92, 221–239. [Google Scholar] [CrossRef]
- Casier, E. Contributions à l’étude des poissons fossiles de la Belgique. XII Sélaciens et Holocéphales sinémuriens de la province de Luxembourg. Bull. R. Belg. Inst. Nat. Sci. 1959, 35, 1–35. [Google Scholar]
- Herman, J. Les Sélaciens des terrains néocrétacés et paléocènes de Belgique et des contrées limitrophes. Eléments d’une biostratigraphie intercontinentale. Mémoires Pour Serv. L’explication Cart. Géologiques Minières Belg. 1977, 15, 1–401. [Google Scholar]
- Glickman, L.S. Class Chondrichthyes, Subclass Elasmobranchii. In Fundamental of Paleontology; Obruchev, D.V., Ed.; Nauka SSSR: Moscow-Leningrad, Russia, 1964; Volume 11, pp. 196–237. [Google Scholar]
- Maisey, J.G. Hamiltonichthys mapesi, g. and sp. nov., (Chondrichthyes, Elasmobranchii) from the Upper Pennsylvanian of Kansas; American Museum of Natural History: New York, NY, USA, 1989; No. 2931; p. 42. [Google Scholar]
- Coates, M.I.; Gess, R.W. A new reconstruction of Onychoselache traquairi, comments on early chondrichtyan pectoral girdles and hybodontiform phylogeny. Palaeontology 2007, 50, 1421–1446. [Google Scholar] [CrossRef]
- Johnson, G.D. Hybodontoidei (Chondrichthyes) from the Wichita-Albany Group (Early Permian) of Texas. J. Vertebr. Paleontol. 1981, 1, 1–41. Available online: https://www.jstor.org/stable/4522833 (accessed on 4 October 2023). [CrossRef]
- Dick, R.F.; Maisey, J.G. Scottish Lower Carboniferous shark Onychoselache traquairi. Palaeontology 1980, 23, 363–374. [Google Scholar]
- Hairapetian, V.; Ginter, M. Famennian chondrichthyan remains from the Chahriseh section, central Iran. Acta Geol. Pol. 2009, 59, 173–200. [Google Scholar]
- Zangerl, R. New chondrichthyes from the Mazon Creek fauna (Pennsylvanian) of Illinois. In Mazon Creek Fossils; Nitecki, M.H., Ed.; Academic Press: New York, NY, USA, 1979; pp. 449–500. [Google Scholar]
- Duffin, C.J. Revision of the hybodont selachian genus Lissodus Brough (1935). Palaontographica Abt. A 1985, 188, 105–152. [Google Scholar]
- Klug, S.; Tutken, T.; Wings, O.; Pfretzschner, H.-U.; Martin, T. A Late Jurassic freshwater shark assemblage (Chondrichthyes, Hybodontiformes) from the southern Junggar Basin, Xinjiang, Northwest China. Palaeobiology Palaeoenvironment 2010, 90, 241–257. [Google Scholar] [CrossRef]
- Ginter, M.; Sun, Y. Chondrichthyan remains from the Lower Carboniferous of Muhua, southern China. Acta Palaeontol. Pol. 2007, 52, 705–727. [Google Scholar]
- Baumiller, T.K.; Gahn, F.J. Predation on Crinoids. In Predator-Prey Interactions in the Fossil Record; Kelley, P.H., Kowalewski, M., Hansen, T.A., Eds.; Springer: Boston, MA, USA, 2003; pp. 263–278. [Google Scholar] [CrossRef]
- Golonka, J.; Gawęda, A. Plate tectonic evolution of the southern margin of Laurussia in the Paleozoic. In Tectonics: Recent Advances; Sharkov, E., Ed.; InTech: Rijeka, Croatia, 2012; pp. 261–282. [Google Scholar]
- Golonka, J. Chapter 6 Phanerozoic palaeoenvironment and palaeolithofacies maps of the Arctic region. In Arctic Petroleum Geology; Spencer, A.M., Embry, A.F., Gautier, D.L., Stoupakova, A.V., Sørensen, K., Eds.; Geological Society, London, Memoirs: London, UK, 2011; Volume 35, pp. 79–129. [Google Scholar]
- Scotese, C.R. Atlas of Earth History; PALEOMAP Project: Arlington, TX, USA, 2001; pp. 1–58. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicimurri, D.; Ciampaglio, C.; Hoenig, M.; Shell, R.; Fuelling, L.; Peterman, D.; Cline, D.A.; Jacquemin, S. A Description of the New Hybodont Shark Genus, Columnaodus, from the Burlington and Keokuk Limestones (Carboniferous, Mississippian, Osagean) of Illinois and Iowa, USA. Diversity 2024, 16, 276. https://doi.org/10.3390/d16050276
Cicimurri D, Ciampaglio C, Hoenig M, Shell R, Fuelling L, Peterman D, Cline DA, Jacquemin S. A Description of the New Hybodont Shark Genus, Columnaodus, from the Burlington and Keokuk Limestones (Carboniferous, Mississippian, Osagean) of Illinois and Iowa, USA. Diversity. 2024; 16(5):276. https://doi.org/10.3390/d16050276
Chicago/Turabian StyleCicimurri, David, Charles Ciampaglio, Matthew Hoenig, Ryan Shell, Lauren Fuelling, David Peterman, Daniel A. Cline, and Stephen Jacquemin. 2024. "A Description of the New Hybodont Shark Genus, Columnaodus, from the Burlington and Keokuk Limestones (Carboniferous, Mississippian, Osagean) of Illinois and Iowa, USA" Diversity 16, no. 5: 276. https://doi.org/10.3390/d16050276




