Gas Sensors Based on Semiconductor Metal Oxides Fabricated by Electrospinning: A Review
Abstract
:1. Introduction
2. Gas Sensor Performance Based on Single SMOs
2.1. The Effect of Adjusting Grain Size on the Sensor Performance
| Materials | Preparation Method | Con (ppm) | OOT (°C) | Response (Ra/Rg) | Refs. |
|---|---|---|---|---|---|
| WO3 nanofibers | Electrospinning | 1 | 200 | 12.523 | [47] |
| WO3 nanorods | Hydrothermal | 1 | 300 | 5.3 | [50] |
| WO3 nanorods | Glancing angle DC magnetron sputtering | 1 | 250 | 9 | [51] |
2.2. The Effect of Increasing the Specific Surface Area on the Sensor Performance
3. Gas Sensor Performance Based on Noble Metal-Modified SMOs
3.1. Modification with Different Noble Metals
3.2. Strategies to Address Catalyst Nanoparticle Agglomeration

4. Gas Sensor Performance Based on Metal Ion-Doped SMOs
4.1. Doping with Transition Metals
4.2. Doping with Rare Earth Metals
4.3. Doping with Metal Cation

5. Gas Sensor Performance Based on Composite SMOs
5.1. Composite SMOs Combined with SMOs or Polymers
5.2. Composite SMOs Combined with Carbon Nanofiber Materials
5.3. Core-Shell Structures and Hierarchical Structures
5.4. Spinel Structure
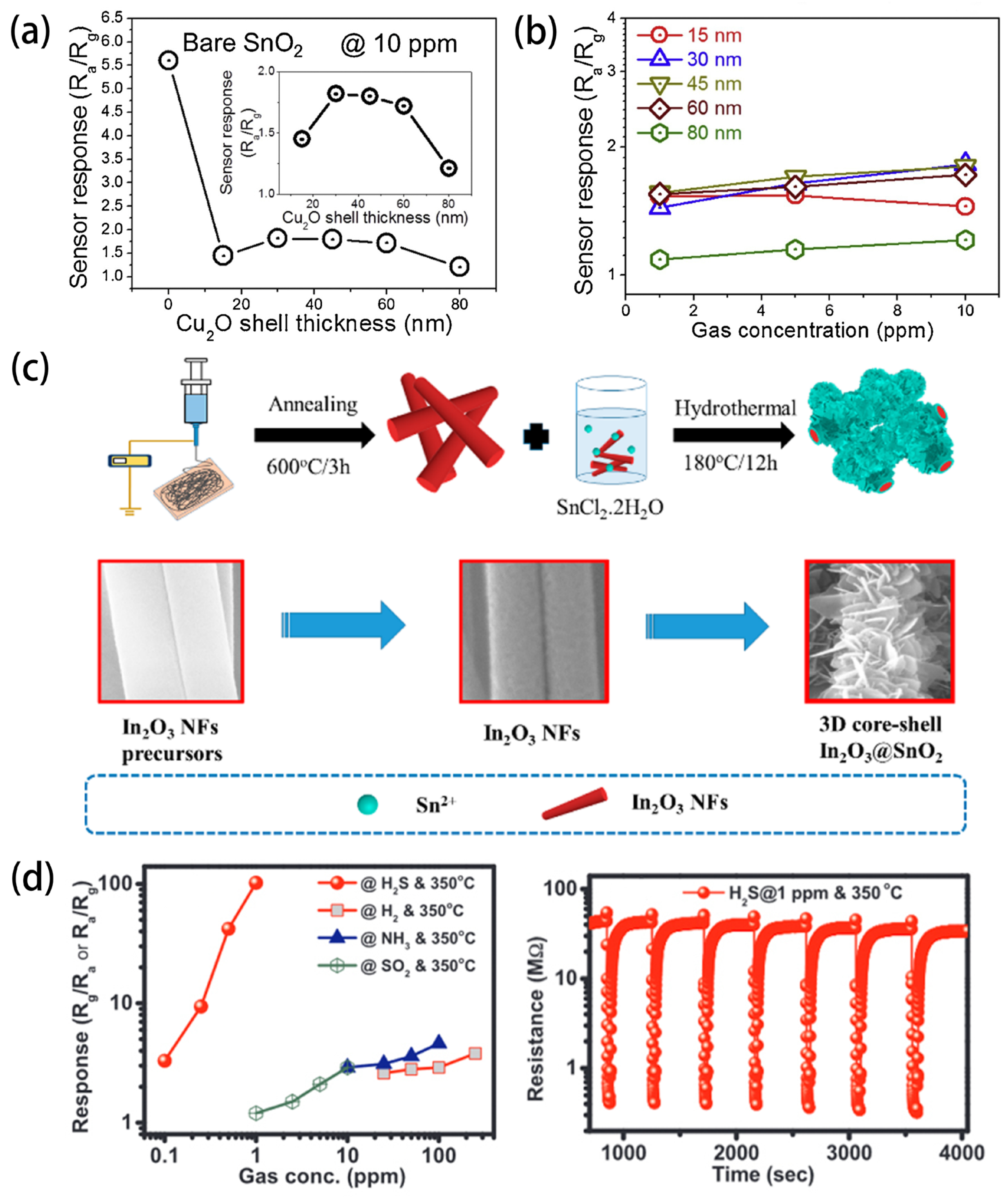
5.5. Perovskite Structures
6. Conclusions and Prospects
- (1)
- In the end, we also make the following predictions for future development and applications. It is worth noting that despite various porous SMO-sensitive materials and sensing mechanisms being reported, poor stability of the sensor remains a significant issue in practical applications. To address this, the use of rare earth elements with high chemical stability as dopants can significantly improve the stability of sensor materials. For example, doping with tungsten may facilitate the desorption of H2O from the surface, improving the humidity resistance of the gas sensor. Furthermore, appropriate functionalization of the surface of SMOs can greatly enhance their stability. Techniques such as coating a protective film on the surface or introducing specific functional groups can effectively mitigate the effects of environmental factors like humidity and temperature changes.
- (2)
- The optimal operating temperature for gas sensors is generally high, leading to increased energy consumption and posing explosion risks in flammable environments. Based on this, there is a need to design gas sensors that operate at room temperature and possess excellent gas sensitivity. Utilizing ultraviolet (UV) light irradiation as an activation energy source is an effective and feasible approach. Thus, exploring the gas sensitivity characteristics of SMOs that are capable of absorbing UV light could lead to progress in this area.
- (3)
- It remains a challenge to select target gases amid various interfering analytes. The sensing mechanism of gas sensors involves processes such as gas adsorption, surface activation, surface reaction, and desorption, closely related to surface catalytic reaction processes. Hence, surface properties like surface acidity/alkalinity, oxygen vacancies, and reactive oxygen species can significantly impact sensing performance and selectivity. Future research should focus more on the catalytic effects of metal oxide-based sensors. Doping with alkali metal elements can significantly adjust surface alkalinity, offering chemical reactivity and excellent selectivity for certain acidic gases. Additionally, employing machine-learning and artificial intelligence algorithms to analyze sensor responses can also improve selectivity. By training models on diverse gas mixtures and sensor responses, it is possible to differentiate between gases with similar physical and chemical properties, thereby enhancing the sensor’s selectivity.
- (4)
- There is still a need to explore novel mesoporous SMO materials (e.g., multi-component and heterojunction materials) and flexible sensing devices to meet practical application requirements. Mesoporous SMO materials with single-atom-doped frameworks, 2D structures, and 1D nanowire heterojunctions hold great potential for developing high-performance miniaturized sensors.
- (5)
- As the demand for miniaturization in electronic devices continues to increase, low operating temperatures and low power consumption are becoming future trends. High-performance MEMS (Micro-Electro-Mechanical System) sensors integrating sensitive layers represent a promising field. Despite this, there is still a need to explore new fabrication techniques to integrate mesoporous SMO materials with MEMS chips to form reliable and stable gas-sensitive nanodevices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Cho, M.; Li, Y.; He, T.; Ahn, J.; Park, J.; Ren, T.L.; Lee, C.; Park, I. Machine learning-enabled textile-based graphene gas sensing with energy harvesting-assisted IoT application. Nano Energy 2021, 86, 106035. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Mirzaei, A.; Navale, S.; Kim, H.W.; Kim, S.S. Boosting the sensing properties of resistive-based gas sensors by irradiation techniques: A review. Nanoscale 2021, 13, 4728–4757. [Google Scholar] [CrossRef] [PubMed]
- Mokrushin, A.S.; Gorban, Y.M.; Averin, A.A.; Gorobtsov, P.Y.; Simonenko, N.P.; Lebedinskii, Y.Y.; Simonenko, E.P.; Kuznetsov, N.T. Obtaining of ZnO/Fe2O3 Thin Nanostructured Films by AACVD for Detection of ppb-Concentrations of NO2 as a Biomarker of Lung Infections. Biosensors 2023, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Chang, Z.; Yuyu, E.; Feng, Y.; Yao, X.; Wang, M.; Ju, Y.; Wang, K.; Jiang, J.; Li, P. Electrospinning and electrospun polysaccharide-based nanofiber membranes: A review. Int. J. Biol. Macromol. 2024, 263, 130335. [Google Scholar] [CrossRef] [PubMed]
- Degler, D.; Weimar, U.; Barsan, N. Current Understanding of the Fundamental Mechanisms of Doped and Loaded Semiconducting Metal-Oxide-Based Gas Sensing Materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.; Xiao, C.; Jia, L. Electrospun Bi-doped SnO2 porous nanosheets for highly sensitive nitric oxide detection. J. Hazard. Mater. 2021, 416, 126118. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, S.-S.; Liu, C.; Chen, L.; Liu, Z.; Guo, Z. Chemiresistive sensor based on hollow Fe2O3 octahedrons incorporated into porous In2O3 nanofibers for enhanced sensing performance and recognition toward triethylamine. Sens. Actuators B Chem. 2023, 393, 134129. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wu, J.; Wang, X.; Lv, X.; Liu, G.; Li, B.; Zhou, J.; Xie, E.; Zhang, Z. Electrospun Nb-doped CeO2 nanofibers for humidity independent acetone sensing. Appl. Surf. Sci. 2022, 602, 154303. [Google Scholar] [CrossRef]
- Naresh, B.; Krishna, K.G.; Rajasekhar, D.; Kuchi, C.; Kummara, S.K.; Reddy, P.S. Synthesis and characterization of rGO wrapped 1-D NiO nanofibers for ammonia gas sensing application. Surf. Interfaces 2023, 40, 103012. [Google Scholar] [CrossRef]
- Das, S.; Mojumder, S.; Saha, D.; Pal, M. Influence of major parameters on the sensing mechanism of semiconductor metal oxide based chemiresistive gas sensors: A review focused on personalized healthcare. Sens. Actuators B Chem. 2022, 352, 131066. [Google Scholar] [CrossRef]
- Gai, L.-Y.; Lai, R.-P.; Dong, X.-H.; Wu, X.; Luan, Q.-T.; Wang, J.; Lin, H.-F.; Ding, W.-H.; Wu, G.-L.; Xie, W.-F. Recent advances in ethanol gas sensors based on metal oxide semiconductor heterojunctions. Rare Met. 2022, 41, 1818–1842. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, Q.; Wang, J.; Lu, Z.; Xu, L.; Zeng, W. Hydrothermal Synthesis of SnO2 Nanoneedle-Anchored NiO Microsphere and its Gas Sensing Performances. Nanomaterials 2019, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, A.; Myung, J.-H. Selective ppb-level NO2 gas sensor based on SnO2-boron nitride nanotubes. Sens. Actuators B Chem. 2021, 331, 129464. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Advancements in improving selectivity of metal oxide semiconductor gas sensors opening new perspectives for their application in food industry. Sensors 2023, 23, 9548. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, D.; Li, T.; Zhang, J.; Yu, L. Green light-driven acetone gas sensor based on electrospinned CdS nanospheres/Co3O4 nanofibers hybrid for the detection of exhaled diabetes biomarker. J. Colloid Interface Sci. 2022, 606, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.S.; Nadargi, D.Y.; Tamboli, M.S.; Alshahrani, T.; Reddy, V.R.M.; Kim, E.S.; Mulla, I.S.; Park, C.; Suryavanshi, S.S. RGO/WO3 hierarchical architectures for improved H2S sensing and highly efficient solar-driving photo-degradation of RhB dye. Sci. Rep. 2021, 11, 5023. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Sun, H.; Li, K.; Xu, W.; He, Q.; Facchetti, A.; Cheng, J. A facile approach for significantly enhancing fluorescent gas sensing by oxygen plasma treatments. Sens. Actuators B Chem. 2021, 331, 129397. [Google Scholar] [CrossRef]
- Saruhan, B.; Fomekong, R.L.; Nahirniak, S. Review: Influences of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Liu, J.; Nasir, M.S.; Zhu, J.; Li, S.; Liang, J.; Yan, W. Smart formaldehyde detection enabled by metal organic framework-derived doped electrospun hollow nanofibers. Sens. Actuators B Chem. 2021, 326, 128819. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Pan, C.; Song, Y.; Dong, H.; Xie, X.; Li, Y.; Liu, J.; Wang, D.; Chen, X. Chemiresistive gas sensors based on electrospun semiconductor metal oxides: A review. Talanta 2022, 246, 123527. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.; Zhang, H.D.; Li, M.M.; Duvail, J.-L.; Jiang, X.; Yin, H. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Lian, S.; Lamprou, D.; Zhao, M. Electrospinning technologies for the delivery of biopharmaceuticals: Current status and future trends. Int. J. Pharm. 2023, 651, 123641. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, É.J.; Kubaski, M.M.; Samara, M.; Zednik, R.J.; Demarquette, N.R. Scaled-Up Multi-Needle Electrospinning Process Using Parallel Plate Auxiliary Electrodes. Nanomaterials 2022, 12, 1356. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthc. Mater. 2019, 9, e1901287. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I.; Czapka, T. Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers 2022, 14, 1661. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, B.; Sun, G.; Wang, M.; Yu, J. Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 2013, 58, 1173–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, S.-H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014. [Google Scholar] [CrossRef]
- Khademi, D.; Ezatpour, H.R.; Huo, Y. The effect of electrospinning time on the structure and mechanical properties of TiO2 continuous nanofibers reinforced aluminum matrix composites. Ceram. Int. 2023, 49, 33804–33817. [Google Scholar] [CrossRef]
- Zhang, B.; Bao, N.; Wang, T.; Xu, Y.; Dong, Y.; Ni, Y.; Yu, P.; Wei, Q.; Wang, J.; Guo, L.; et al. High-performance room temperature NO2 gas sensor based on visible light irradiated In2O3 nanowires. J. Alloys Compd. 2021, 867, 159076. [Google Scholar] [CrossRef]
- Loloei, Y.H.; Mehdikhani, M.; Askari, V.; Kharazi, A.Z. Electrospun core-shell polycaprolactone/chitosan nanofibrous composite with enhanced curcumin loading capacity for wound healing applications. Mater. Today Chem. 2024, 36, 101956. [Google Scholar] [CrossRef]
- Li, J.; Xian, J.; Wang, W.; Cheng, K.; Zeng, M.; Zhang, A.; Wu, S.; Gao, X.; Lu, X.; Liu, J.-M. Ultrafast response and high-sensitivity acetone gas sensor based on porous hollow Ru-doped SnO2 nanotubes. Sens. Actuators B Chem. 2022, 352, 131061. [Google Scholar] [CrossRef]
- Cheng, R.; Liang, Z.; Shen, H.; Guo, J.; Wang, C.-F.; Chen, S. In-situ synthesis of stable perovskite quantum dots in core-shell nanofibers via microfluidic electrospinning. Chin. Chem. Lett. 2023, 34, 107384. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Dvorak, L.; Yalcinkaya, F. Chemical Cleaning Process of Polymeric Nanofibrous Membranes. Polymers 2022, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, X.; Zhu, Y.; Cheng, X.; Zhao, D.; Deng, Y. sp2-Hybridized Carbon-Containing Block Copolymer Templated Synthesis of Mesoporous Semiconducting Metal Oxides with Excellent Gas Sensing Property. Acc. Chem. Res. 2019, 52, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.V.; Suman, P.H.; Silva, R.A.; Orlandi, M.O. High gas sensor performance of WO3 nanofibers prepared by electrospinning. J. Alloys Compd. 2021, 864, 158745. [Google Scholar] [CrossRef]
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Patil, S.P.; Harale, N.S.; Bhosale, J.L.; Kim, J.H.; Patil, P.S. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B Chem. 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Sheng, K.; Chen, C.; Zhou, X.; Dong, B.; Bai, X.; Zhang, S.; Lu, G.; Song, H. APTES-functionalized thin-walled porous WO3 nanotubes for highly selective sensing of NO2 in a polluted environment. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 10976–10989. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y. Synthesis, growth kinetics and ultra-sensitive performance of electrospun WO3 nanofibers for NO2 detection. Appl. Surf. Sci. 2023, 608, 155112. [Google Scholar] [CrossRef]
- Ezhilan, M.; Jbb, A.J.; Rayappan, J.B.B. Influence of PVA templates on the synthesis of interconnected and long-winded electrospun V2O5 nanowires—Acetone sensor. Mater. Res. Bull. 2021, 139, 111276. [Google Scholar] [CrossRef]
- Che, Y.; Feng, G.; Sun, T.; Xiao, J.; Guo, W.; Song, C. Excellent gas-sensitive properties towards acetone of In2O3 nanowires prepared by electrospinning. Colloid Interface Sci. Commun. 2021, 45, 100508. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Wang, Y.; Hu, Y.; Yu, X.; Zhang, G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012, 2, 8525–8531. [Google Scholar] [CrossRef]
- Horprathum, M.; Limwichean, K.; Wisitsoraat, A.; Eiamchai, P.; Aiempanakit, K.; Limnonthakul, P.; Nuntawong, N.; Pattantsetakul, V.; Tuantranont, A.; Chindaudom, P. NO2-sensing properties of WO3 nanorods prepared by glancing angle DC magnetron sputtering. Sens. Actuators B Chem. 2013, 176, 685–691. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Hsu, Y.-W. Enhanced Sensing Ability of Brush-Like Fe2O3-ZnO Nanostructures towards NO2 Gas via Manipulating Material Synergistic Effect. Int. J. Mol. Sci. 2021, 22, 6884. [Google Scholar] [CrossRef] [PubMed]
- Kgomo, M.B.; Shingange, K.; Nemufulwi, M.I.; Swart, H.C.; Mhlongo, G.H. Belt-like In2O3 based sensor for methane detection: Influence of morphological, surface defects and textural behavior. Mater. Res. Bull. 2023, 158, 112076. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Li, G.; Bian, X.; Liu, Y.; Zhang, J.; Gao, J.; Wang, C.; Zhu, B.; Lu, H. Metal–organic framework-derived CuO tube-like nanofibers with high surface area and abundant porosities for enhanced room-temperature NO2 sensing properties. J. Alloys Compd. 2023, 934, 167950. [Google Scholar] [CrossRef]
- Giancaterini, L.; Emamjomeh, S.M.; De Marcellis, A.; Palange, E.; Resmini, A.; Anselmi-Tamburini, U.; Cantalini, C. The influence of thermal and visible light activation modes on the NO2 response of WO3 nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2016, 229, 387–395. [Google Scholar] [CrossRef]
- Ezhilan, M.; Jbb, A.J.; Babu, K.J.; Rayappan, J.B.B. Hierarchically connected electrospun WO3 nanowires—An acetaldehyde sensor. J. Alloys Compd. 2021, 863, 158407. [Google Scholar] [CrossRef]
- Zhang, J.; Leng, D.; Zhang, L.; Li, G.; Ma, F.; Gao, J.; Lu, H.; Zhu, B. Porosity and oxygen vacancy engineering of mesoporous WO3 nanofibers for fast and sensitive low-temperature NO2 sensing. J. Alloys Compd. 2021, 853, 157339. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, G.; Du, W.; Tian, Q. Synthesis and excellent acetone sensing properties of porous WO3 nanofibers. Vacuum 2016, 124, 32–39. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Hung, C.M.; Van Toan, N.; Van Duy, N.; Hoa, N.D.; Van Hieu, N. One-step fabrication of SnO2 porous nanofiber gas sensors for sub-ppm H2S detection. Sens. Actuators A Phys. 2020, 303, 111722. [Google Scholar] [CrossRef]
- Su, P.; Li, W.; Zhang, J.; Xie, X. Chemiresistive gas sensor based on electrospun hollow SnO2 nanotubes for detecting NO at the ppb level. Vacuum 2022, 199, 110961. [Google Scholar] [CrossRef]
- Islam, M.; Srivastava, A.K.; Basavaraja, B.M.; Sharma, A. “Nano-on-Micro” approach enables synthesis of ZnO nano-cactus for gas sensing applications. Sens. Int. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Kishore, K.R.; Balamurugan, D.; Jeyaprakash, B.G. Electrospinning based CdO nanograins for formaldehyde vapour detection by chemiresistive method. Mater. Sci. Semicond. Process. 2021, 121, 105296. [Google Scholar] [CrossRef]
- Busacca, C.; Donato, A.; Faro, M.L.; Malara, A.; Neri, G.; Trocino, S. CO gas sensing performance of electrospun Co3O4 nanostructures at low operating temperature. Sens. Actuators B Chem. 2020, 303, 127193. [Google Scholar] [CrossRef]
- Wang, T.; Can, I.; Zhang, S.; He, J.; Sun, P.; Liu, F.; Lu, G. Self-Assembly Template Driven 3D Inverse Opal Microspheres Functionalized with Catalyst Nanoparticles Enabling a Highly Efficient Chemical Sensing Platform. ACS Appl. Mater. Interfaces 2018, 10, 5835–5844. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, X.; Xue, D.; Zong, F.; Zhang, J.; Duan, X.; Li, Q.; Wang, T. Enhanced H2 gas sensing properties by Pd-loaded urchin-like W18O49 hierarchical nanostructures. Sens. Actuators B Chem. 2018, 260, 900–907. [Google Scholar] [CrossRef]
- Rong, Q.; Xiao, B.L.; Zeng, J.; Yu, R.; Zi, B.; Zhang, G.; Zhu, Z.; Zhang, J.; Wu, J.; Liu, Q. Pt Single Atom-Induced Activation Energy and Adsorption Enhancement for an Ultrasensitive ppb-Level Methanol Gas Sensor. ACS Sens. 2021, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xie, N.; Wang, C.; Kou, X.; Ding, M.; Zhang, H.; Sun, Y.; Song, H.; Wang, Y.; Lu, G. Enhanced hydrogen sulfide sensing properties of Pt-functionalized α-Fe2O3 nanowires prepared by one-step electrospinning. Sens. Actuators B Chem. 2018, 255, 1015–1023. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Li, F.; Lu, G.; Zhang, T.; Barsan, N. Pt-In2O3 mesoporous nanofibers with enhanced gas sensing performance towards ppb-level NO2 at room temperature. Sens. Actuators B Chem. 2018, 260, 927–936. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.-H.; Kim, I.-D. Controlled synthesis of electrospun hollow Pt-loaded SnO2 microbelts for acetone sensing. Sens. Actuators B Chem. 2021, 344, 130208. [Google Scholar] [CrossRef]
- Teng, L.; Liu, Y.; Ikram, M.; Liu, Z.; Ullah, M.; Ma, L.; Zhang, X.; Wu, H.; Li, L.; Shi, K. One-step synthesis of palladium oxide-functionalized tin dioxide nanotubes: Characterization and high nitrogen dioxide gas sensing performance at room temperature. J. Colloid Interface Sci. 2018, 537, 79–90. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, B.; Li, Y.; Zhang, S.; Zhang, Y.; Hua, X.; Liu, G.; Li, B.; Zhou, J.; Xie, E.; et al. Methanol gas detection of electrospun CeO2 nanofibers by regulating Ce3+/Ce4+ mole ratio via Pd doping. Sens. Actuators B Chem. 2020, 307, 127638. [Google Scholar] [CrossRef]
- Jaroenapibal, P.; Boonma, P.; Saksilaporn, N.; Horprathum, M.; Amornkitbamrung, V.; Triroj, N. Improved NO2 sensing performance of electrospun WO3 nanofibers with silver doping. Sens. Actuators B Chem. 2018, 255, 1831–1840. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Y.; Zhang, C.; Yu, H.; Wang, T.; Shi, K.; Zhang, Z.; Wang, D.; Dong, X. High selectivity of Ag-doped Fe2O3 hollow nanofibers in H2S detection at room operating temperature. Sens. Actuators B Chem. 2021, 341, 129919. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Hosseini, S.; Salehifar, N. Fabrication of a Highly Sensitive Single Aligned TiO2 and Gold Nanoparticle Embedded TiO2 Nano-Fiber Gas Sensor. ACS Appl. Mater. Interfaces 2017, 9, 15662–15671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Y.; Wang, M.; Ma, S.; Wang, P.; Zhang, G.; Chen, W.; Jiao, H.; Liu, L.; Xu, X. Enhanced acetone sensor based on Au functionalized In-doped ZnSnO3 nanofibers synthesized by electrospinning method. J. Colloid Interface Sci. 2019, 543, 285–299. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Chen, J.; Zhang, L.; Zhang, Y.; Jin, P. Au modified PrFeO3 with hollow tubular structure can be efficient sensing material for H2S detection. Front. Bioeng. Biotechnol. 2022, 10, 969870. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-I.; Hwang, S.-J.; Dai, Z.; Kang, Y.C.; Lee, J.-H. Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics. RSC Adv. 2014, 4, 53130–53136. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Wang, L.; Wang, B.; Liu, F.; Liang, X.; Sun, P.; Yan, X.; Chuai, X.; Lu, G. Improvement of NO2 sensing characteristic for mixed potential type gas sensor based on YSZ and Rh/Co3V2O8 sensing electrode. RSC Adv. 2017, 7, 49440–49445. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Nehasil, V. The role of Rh dispersion in gas sensing effects observed in SnO2 thin films. Mater. Chem. Phys. 2019, 232, 160–168. [Google Scholar] [CrossRef]
- Li, Z.; Lou, C.; Lei, G.; Lu, G.; Pan, H.; Liu, X.; Zhang, J. Atomic layer deposition of Rh/ZnO nanostructures for anti-humidity detection of trimethylamine. Sens. Actuators B Chem. 2022, 355, 131347. [Google Scholar] [CrossRef]
- Sun, N.; Tian, Q.; Bian, W.; Wang, X.; Dou, H.; Li, C.; Zhang, Y.; Gong, C.; You, X.; Du, X.; et al. Highly sensitive and lower detection-limit NO2 gas sensor based on Rh-doped ZnO nanofibers prepared by electrospinning. Appl. Surf. Sci. 2023, 614, 156213. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S.; Tuller, H.L.; Kim, I.-D. Heterogeneous Sensitization of Metal–Organic Framework Driven Metal@Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef]
- Yoon, K.R.; Lee, G.Y.; Jung, J.-W.; Kim, N.-H.; Kim, S.O.; Kim, I.-D. One-Dimensional RuO2/Mn2O3 Hollow Architectures as Efficient Bifunctional Catalysts for Lithium–Oxygen Batteries. Nano Lett. 2016, 16, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kim, J.; Yoon, K.R.; Jung, J.-W.; Oh, J.; Kim, I.-D. Rational Design of Efficient Electrocatalysts for Hydrogen Evolution Reaction: Single Layers of WS2 Nanoplates Anchored to Hollow Nitrogen-Doped Carbon Nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 28116–28121. [Google Scholar] [CrossRef]
- Ma, L.; Fan, H.; Wang, J.; Zhao, Y.; Tian, H.; Dong, G. Water-assisted ions in situ intercalation for porous polymeric graphitic carbon nitride nanosheets with superior photocatalytic hydrogen evolution performance. Appl. Catal. B Environ. 2016, 190, 93–102. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Ma, J.; Ma, L.; Dong, G. Noble metal-free modified electrode of exfoliated graphitic carbon nitride/ZnO nanosheets for highly efficient hydrogen peroxide sensing. Electrochim. Acta 2017, 247, 787–794. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jang, J.-S.; Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Kim, I.-D. Hierarchically interconnected porosity control of catalyst-loaded WO3 nanofiber scaffold: Superior acetone sensing layers for exhaled breath analysis. Sens. Actuators B Chem. 2018, 259, 616–625. [Google Scholar] [CrossRef]
- Jang, J.-S.; Yu, S.; Choi, S.-J.; Kim, S.-J.; Koo, W.-T.; Kim, I.-D. Metal Chelation Assisted In Situ Migration and Functionalization of Catalysts on Peapod-Like Hollow SnO2 toward a Superior Chemical Sensor. Small 2016, 12, 5989–5997. [Google Scholar] [CrossRef]
- Choi, S.-J.; Ku, K.H.; Kim, B.J.; Kim, I.-D. Novel Templating Route Using Pt Infiltrated Block Copolymer Microparticles for Catalytic Pt Functionalized Macroporous WO3 Nanofibers and Its Application in Breath Pattern Recognition. ACS Sens. 2016, 1, 1124–1131. [Google Scholar] [CrossRef]
- Kou, X.; Xie, N.; Chen, F.; Wang, T.; Guo, L.; Wang, C.; Wang, Q.; Ma, J.; Sun, Y.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B Chem. 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Bai, J.; Luo, Y.; An, B.; Wang, Q.; Cheng, X.; Li, J.; Pan, X.; Zhou, J.; Wang, Y.; Xie, E. Ni/Au bimetal decorated In2O3 nanotubes for ultra-sensitive ethanol detection. Sens. Actuators B Chem. 2020, 311, 127938. [Google Scholar] [CrossRef]
- Luo, Y.; An, B.; Bai, J.; Wang, Y.; Cheng, X.; Wang, Q.; Li, J.; Yang, Y.; Wu, Z.; Xie, E. Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Koo, W.-T.; Jang, J.-S.; Kim, D.-H.; Kim, M.-H.; Kim, I.-D. Nanoscale PtO2 Catalysts-Loaded SnO2 Multichannel Nanofibers toward Highly Sensitive Acetone Sensor. ACS Appl. Mater. Interfaces 2018, 10, 2016–2025. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, H.; Hu, J.; Lv, T.; Rong, Q.; Zhang, Y.; Zi, B.; Chen, M.; Zhang, D.; Wei, J.; et al. Formaldehyde gas sensor with extremely high response employing cobalt-doped SnO2 ultrafine nanoparticles. Nanoscale Adv. 2022, 4, 824. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, Q.; Wang, Y.; Cheng, X.; Yang, Z.; Gu, X.; Huang, B.; Sun, G.; Zhang, Z.; Pan, X.; et al. Role of nickel dopant on gas response and selectivity of electrospun indium oxide nanotubes. J. Colloid Interface Sci. 2020, 560, 447–457. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wei, W.; Jiang, H.; Li, X.; Liu, G.; Zhu, Z.; Li, B.; Sheng, Y.; Zhou, J.; et al. Humidity-resistant ethanol gas sensors based on electrospun tungsten-doped cerium oxide hollow nanofibers. Sens. Actuators B Chem. 2023, 393, 134210. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Shang, H.; Wu, J. Graphene Quantum Dots Modified Upconversion Nanoparticles for Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12558. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.; Xu, L.; Zhu, S.; Zhou, X.; Yang, S.; Dong, B.; Bai, X.; Lu, G.; Song, H. Understanding the noble metal modifying effect on In2O3 nanowires: Highly sensitive and selective gas sensors for potential early screening of multiple diseases. Nanoscale Horiz. 2019, 4, 1361–1371. [Google Scholar] [CrossRef]
- Huang, G.; Duan, L.; Dong, G.; Zhang, D.; Qiu, Y. High-Mobility Solution-Processed Tin Oxide Thin-Film Transistors with High-κ Alumina Dielectric Working in Enhancement Mode. ACS Appl. Mater. Interfaces 2014, 6, 20786–20794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, F.; Han, D.; Wang, Z.; Guo, G. Synthesis, characterization and alcohol-sensing properties of rare earth doped In2O3 hollow spheres. Sens. Actuators B Chem. 2013, 177, 1180–1188. [Google Scholar] [CrossRef]
- Jun, L.; Chen, Q.; Fu, W.; Yang, Y.; Zhu, W.; Zhang, J. Electrospun Yb-Doped In2O3 Nanofiber Field-Effect Transistors for Highly Sensitive Ethanol Sensors. ACS Appl. Mater. Interfaces 2020, 12, 38425–38434. [Google Scholar] [CrossRef]
- De Pascali, C.; Signore, M.A.; Taurino, A.; Francioso, L.; Macagnano, A.; Avossa, J.; Siciliano, P.; Capone, S. Investigation of the Gas-Sensing Performance of Electrospun TiO2 Nanofiber-Based Sensors for Ethanol Sensing. IEEE Sens. J. 2018, 18, 7365–7374. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Y.; Hu, Q.; Mu, X.; Zhang, Y.; Bai, J.; Wang, Q.; Sheng, Y.; Zhang, Z.; Xie, E. A low temperature and highly sensitive ethanol sensor based on Au modified In2O3 nanofibers by coaxial electrospinning. J. Mater. Chem. C 2018, 6, 10935–10943. [Google Scholar] [CrossRef]
- Tie, Y.; Ma, S.; Pei, S.; Zhang, Q.; Zhu, K.; Zhang, R.; Xu, X.; Han, T.; Liu, W. Pr doped BiFeO3 hollow nanofibers via electrospinning method as a formaldehyde sensor. Sens. Actuators B Chem. 2020, 308, 127689. [Google Scholar] [CrossRef]
- Yu, S.; Jia, X.; Yang, J.; Wang, S.; Li, Y.; Song, H. Highly sensitive and low detection limit of ethanol gas sensor based on CeO2 nanodot-decorated ZnSnO3 hollow microspheres. Ceram. Int. 2022, 48, 14865–14875. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, R.; Sun, B.; Nie, G.; Ji, H.; Lei, J.; Wang, C. Highly sensitive acetone sensor based on Eu-doped SnO2 electrospun nanofibers. Ceram. Int. 2016, 42, 15881–15888. [Google Scholar] [CrossRef]
- Liang, Q.; Qu, X.; Bai, N.; Chen, H.; Zou, X.; Li, G.-D. Alkali metal-incorporated spinel oxide nanofibers enable high performance detection of formaldehyde at ppb level. J. Hazard. Mater. 2020, 400, 123301. [Google Scholar] [CrossRef]
- Zhao, C.; Bai, J.; Huang, B.; Wang, Y.; Zhou, J.; Xie, E. Grain refining effect of calcium dopants on gas-sensing properties of electrospun α-Fe2O3 nanotubes. Sens. Actuators B Chem. 2016, 231, 552–560. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, C.; Yang, L.; Zhang, S.; Zhang, G.; Cai, Z. Enhanced gas sensing performance of Li-doped ZnO nanoparticle film by the synergistic effect of oxygen interstitials and oxygen vacancies. Appl. Surf. Sci. 2015, 330, 126–133. [Google Scholar] [CrossRef]
- Pramod, N.; Pandey, S. Effect of Li doping on the structural, optical and formaldehyde sensing properties of In2O3 thin films. Ceram. Int. 2015, 41, 527–532. [Google Scholar] [CrossRef]
- Luo, Q.; Wu, J.; Zou, S.; Wang, W.; Wang, Z.; Wan, Y.; Feng, C. N-Butanol sensor based on electrospun Al doped ZnFe2O4 nanofibers. J. Taiwan Inst. Chem. Eng. 2023, 145, 104820. [Google Scholar] [CrossRef]
- Zou, C.; Liang, F.; Xue, S. Synthesis and oxygen vacancy related NO2 gas sensing properties of ZnO:Co nanorods arrays gown by a hydrothermal method. Appl. Surf. Sci. 2015, 353, 1061–1069. [Google Scholar] [CrossRef]
- Zhao, C.; Gong, H.; Niu, G.; Wang, F. Electrospun Ca-doped In2O3 nanotubes for ethanol detection with enhanced sensitivity and selectivity. Sens. Actuators B Chem. 2019, 299, 126946. [Google Scholar] [CrossRef]
- Tian, X.; Yao, L.; Cui, X.; Zhao, R.; Xiao, X.; Wang, Y. Novel Al-doped CdIn2O4 nanofibers based gas sensor for enhanced low-concentration n-butanol sensing. Sens. Actuators B Chem. 2022, 351, 130946. [Google Scholar] [CrossRef]
- Cai, Z.; Park, J.; Park, S. Porous In2O3–ZnO nanofiber-based sensor for ultrasensitive room-temperature detection of toluene gas under UV illumination. J. Mater. Res. Technol. 2023, 24, 2482–2499. [Google Scholar] [CrossRef]
- Beniwal, A. Sunny Novel TPU/Fe2O3 and TPU/Fe2O3/PPy nanocomposites synthesized using electrospun nanofibers investigated for analyte sensing applications at room temperature. Sens. Actuators B Chem. 2020, 304, 127384. [Google Scholar] [CrossRef]
- Li, C.; Choi, P.G.; Masuda, Y. Highly Sensitive and Selective Gas Sensors Based on NiO/MnO2@NiO Nanosheets to Detect Allyl Mercaptan Gas Released by Humans under Psychological Stress. Adv. Sci. 2022, 9, e2202442. [Google Scholar] [CrossRef]
- Song, L.; Yang, L.; Wang, Z.; Liu, D.; Luo, L.; Zhu, X.; Xi, Y.; Yang, Z.; Han, N.; Wang, F.; et al. One-step electrospun SnO2/MOx heterostructured nanomaterials for highly selective gas sensor array integration. Sens. Actuators B Chem. 2019, 283, 793–801. [Google Scholar] [CrossRef]
- Kumar, A.; Shringi, A.K.; Kumar, M. RF sputtered CuO anchored SnO2 for H2S gas sensor. Sens. Actuators B Chem. 2022, 370, 132417. [Google Scholar] [CrossRef]
- Navale, S.; Liu, C.; Gaikar, P.; Patil, V.; Sagar, R.; Du, B.; Mane, R.; Stadler, F. Solution-processed rapid synthesis strategy of Co3O4 for the sensitive and selective detection of H2S. Sens. Actuators B Chem. 2017, 245, 524–532. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Gao, Y.; Chen, F.; Wang, T.; Xia, H.; Sui, X.; Wang, Z. Fast-response hydrogen sulfide gas sensor based on electrospinning Co3O4 nanofibers-modified CuO nanoflowers: Experimental and DFT calculation. Sens. Actuators B Chem. 2023, 396, 134579. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent Advances in Sensing Applications of Graphene Assemblies and Their Composites. Adv. Funct. Mater. 2017, 27, 2891. [Google Scholar] [CrossRef]
- Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T. Chemiresistive Graphene Sensors for Ammonia Detection. ACS Appl. Mater. Interfaces 2018, 10, 16169–16176. [Google Scholar] [CrossRef]
- Yan, C.; Lu, H.; Gao, J.; Zhang, Y.; Guo, Q.; Ding, H.; Wang, Y.; Wei, F.; Zhu, G.; Yang, Z.; et al. Improved NO2 sensing properties at low temperature using reduced graphene oxide nanosheet–In2O3 heterojunction nanofibers. J. Alloys Compd. 2018, 741, 908–917. [Google Scholar] [CrossRef]
- Guo, L.; Kou, X.; Ding, M.; Wang, C.; Dong, L.; Zhang, H.; Feng, C.; Sun, Y.; Gao, Y.; Sun, P.; et al. Reduced graphene oxide/α-Fe2O3 composite nanofibers for application in gas sensors. Sens. Actuators B Chem. 2017, 244, 233–242. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Zheng, Y.; Lee, J.-H.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Enhancement of H2S sensing performance of p-CuO nanofibers by loading p-reduced graphene oxide nanosheets. Sens. Actuators B Chem. 2019, 281, 453–461. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Mo, J.; Deng, B.; Wang, J.; Zhu, Y.; Xiao, X.; Xu, G. Construction of hierarchical structure of Co3O4 electrode based on electrospinning technique for supercapacitor. J. Alloys Compd. 2021, 853, 157271. [Google Scholar] [CrossRef]
- Jian, W.; Ye, Z.; Yipeng, Z.; Hui, Z. Stable encapsulation of camellia oil in core–shell zein nanofibers fabricated by emulsion electrospinning. Food Chem. 2023, 429, 136860. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.W.; Kim, S.S. Ultra-sensitive benzene detection by a novel approach: Core-shell nanowires combined with the Pd-functionalization. Sens. Actuators B Chem. 2017, 239, 578–585. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Enhancement of CO and NO2 sensing in n-SnO2-p-Cu2O core-shell nanofibers by shell optimization. J. Hazard. Mater. 2019, 376, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 core-shell nanofibers and their enhanced gas sensing performance based on different work function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Fang, Y.; Wang, J.; Che, Q.; Wang, G.; Yang, P. Novel Construction of Morphology-Tunable C–N/SnO2/ZnO/Au Microspheres with Ultrasensitivity and High Selectivity for Triethylamine under Various Temperature Detections. ACS Appl. Mater. Interfaces 2019, 11, 8601–8611. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Li, H.; Che, Q.; Wang, J.; Wang, G.; Yang, P. Multilevel Effective Heterojunctions Based on SnO2/ZnO 1D Fibrous Hierarchical Structure with Unique Interface Electronic Effects. ACS Appl. Mater. Interfaces 2019, 11, 31551–31561. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Wang, D.; Wang, F.; Li, H.; Xu, J.; Wang, X.; Yang, J. Hierarchical In2O3@SnO2 Core–Shell Nanofiber for High Efficiency Formaldehyde Detection. ACS Appl. Mater. Interfaces 2019, 11, 45214–45225. [Google Scholar] [CrossRef] [PubMed]
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Deepty, M.; Srinivas, C.; Ranjith Kumar, E.; Ramesh, P.N.; Krishna Mohan, N.; Meena, S.S.; Prajapat, C.; Verma, A.; Sastry, D. Evaluation of structural and dielectric properties of Mn2+-substituted Zn-spinel ferrite nanoparticles for gas sensor applications. Sens. Actuators B Chem. 2020, 316, 128127. [Google Scholar] [CrossRef]
- Mkwae, P.S.; Kortidis, I.; Kroon, R.E.; Leshabane, N.; Jozela, M.; Swart, H.C.; Nkosi, S.S. Insightful acetone gas sensing behaviour of Ce substituted MgFe2O4 spinel nano-ferrites. J. Mater. Res. Technol. 2020, 9, 16252–16269. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Guo, H.; Sun, P.; Liu, F.; Liang, X.; Lu, G. Double-Shell Architectures of ZnFe2O4 Nanosheets on ZnO Hollow Spheres for High-Performance Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 17811–17818. [Google Scholar] [CrossRef]
- Van Hoang, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Van Hieu, N. Facile on-chip electrospinning of ZnFe2O4 nanofiber sensors with excellent sensing performance to H2S down ppb level. J. Hazard. Mater. 2018, 360, 6–16. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Van Hoang, N.; Hung, N.M.; Hung, P.T.; Hoat, P.D.; Van Hieu, N. On-chip CuFe2O4 nanofiber for conductometric NO2 and H2S gas-sensors. Sens. Actuators B Chem. 2023, 380, 133306. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, W.; Li, Y.; Yang, X.; Sun, P.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; Ma, J.; et al. Highly-sensitivity acetone sensors based on spinel-type oxide (NiFe2O4) through optimization of porous structure. Sens. Actuators B Chem. 2019, 291, 266–274. [Google Scholar] [CrossRef]
- Van Hoang, N.; Duc, L.M.; Hiep, N.T.; Hung, N.M.; Nguyen, C.V.; Hung, P.T.; Hoat, P.D.; Vo, V.K.; Heo, Y.-W. Optimization of synthesis conditions and sensing performance of electrospun NiFe2O4 nanofibers for H2S and NO2 detection. J. Alloys Compd. 2023, 936, 168276. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Structure, morphology and electrochemical properties of LaxSr1−xCo0.1Mn0.9O3−δ perovskite nanofibers prepared by electrospinning method. J. Alloys Compd. 2015, 624, 31–39. [Google Scholar] [CrossRef]
- Syue, Y.-K.; Hsu, K.-C.; Fang, T.-H.; Lee, C.-I.; Shih, C.-J. Characteristics and gas sensor applications of ZnO-Perovskite heterostructure. Ceram. Int. 2022, 48, 12585–12591. [Google Scholar] [CrossRef]
- Mun, T.; Koo, J.Y.; Lee, J.; Kim, S.J.; Umarji, G.; Amalnerkar, D.; Lee, W. Resistive-type lanthanum ferrite oxygen sensor based on nanoparticle-assimilated nanofiber architecture. Sens. Actuators B Chem. 2020, 324, 128712. [Google Scholar] [CrossRef]
- Zhu, M.; Miao, J.; Duan, X.; Guan, D.; Zhong, Y.; Wang, S.; Zhou, W.; Shao, Z. Postsynthesis Growth of CoOOH Nanostructure on SrCo0.6Ti0.4O3−δ Perovskite Surface for Enhanced Degradation of Aqueous Organic Contaminants. ACS Sustain. Chem. Eng. 2018, 6, 15737–15748. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, H.; Zhang, H.; Lü, W.; Hu, J. CO2 gas sensors based on Yb1−xCaxFeO3 nanocrystalline powders. J. Rare Earths 2017, 35, 602–609. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.; Zhang, P.; Hu, J. High Sensing Properties of 3 wt % Pd-Doped SmFe1–xMgxO3 Nanocrystalline Powders to Acetone Vapor with Ultralow Concentrations under Light Illumination. ACS Appl. Mater. Interfaces 2018, 10, 15558–15564. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.; Xu, X.; Cao, P.; Liu, W.; Xu, X.; Pei, S. Electrospinning synthesis, crystal structure, and ethylene glycol sensing properties of orthorhombic SmBO3 (B=Fe, Co) perovskites. J. Alloys Compd. 2021, 876, 160211. [Google Scholar] [CrossRef]
- Queraltó, A.; Graf, D.; Frohnhoven, R.; Fischer, T.; Vanrompay, H.; Bals, S.; Bartasyte, A.; Mathur, S. LaFeO3 Nanofibers for High Detection of Sulfur-Containing Gases. ACS Sustain. Chem. Eng. 2019, 7, 6023–6032. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Wang, X.; Li, L.; Hu, J. Acetone sensing properties and mechanism of nano-LaFeO3 thick-films. Sens. Actuators B Chem. 2016, 235, 56–66. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.; Shen, X.; Wang, T.; Jiang, X.; Chen, Q.; Qiang, Z.; Yang, H.; Chen, H. PrFeO3 hollow nanofibers as a highly efficient gas sensor for acetone detection. Sens. Actuators B Chem. 2018, 255, 2546–2554. [Google Scholar] [CrossRef]
- Wei, J.S.; Ma, S.Y.; Cai, Y.H.; Xu, C.Y.; Liu, J.M.; Jiang, H.T. A high-performance ethylene glycol sensor based on fibrous ErFeO3 prepared by electrostatic spinning. Ceram. Int. 2023, 49, 32611–32618. [Google Scholar] [CrossRef]
- Yilmaz, O.E.; Erdem, R. Evaluating hydrogen detection performance of an electrospun CuZnFe2O4 nanofiber sensor. Int. J. Hydrog. Energy 2020, 45, 26402–26412. [Google Scholar] [CrossRef]






| Materials | Morphology | Target Gas | D (nm) | OOT (°C) | Con (ppm) | Response (Ra/Rg) | Response Time (s) | Recovery Time (s) | LOD (ppm) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| WO3 | Nanofibers | NO2 | 59–65 | 150 | 25 | 15,000 (Rg/Ra) | 900 | 48 | 0.5 | [44] |
| WO3 | Nanofibers | NO2 | 100–200 | 200 | 1 | 12.52 (Rg/Ra) | 11 | 26 | 0.2 | [47] |
| WO3 | Nanofibers | NO2 | 20–100 | 75 | 0.4 | 12.46 (Rg/Ra) | 1980 | 2280 | 0.1 | [55] |
| WO3 | Nanowires | CH3CHO | 100 | 30 | 75 | 5537 | 49 | 76 | 0.75 | [56] |
| WO3 | Nanofibers | NO2 | 30.56 | 90 | 3 | 101.3 | 125 | 231 | 0.1 | [57] |
| WO3 | Nanofibers | C2H5OH | 275 | 270 | 50 | 55.6 | 6–13 | 4–9 | 0.1 | [58] |
| In2O3 | Nanowires | NO2 | 50 | 25 | 5 | 740 (Rg/Ra) | 200 | 20 | 0.01 | [38] |
| In2O3 | Nanowires | C2H5OH | 100 | 200 | 100 | 37.9 | 1 | 7 | 5 | [49] |
| In2O3 | Belt-like | CH4 | 14 | 100 | 90 | 1.1 | 36 | 44 | 0.18 | [53] |
| SnO2 | Nanofiber | H2S | 150 | 350 | 0.1–1 | 15.2 | 15 | 230 | 0.0016 | [59] |
| SnO2 | Nanotubes | NO | 25.7 | 160 | 0.5 | 33.3 (Rg/Ra) | 214 | 115 | 0.01 | [60] |
| ZnO | Nano-cactus | C2H5OH | 709 ± 62.08 | 230 | 500 | 20.23 (Rg/Ra) | 4700 | 5000 | 10 | [61] |
| V2O5 | Nanowires | C3H6O | 80 | 30 | 50 | 97 | 49 | 19 | 1 | [48] |
| CdO | Nanograins | CH2O | 70 | 350 | 100 | 82 | 43 | 23 | 5 | [62] |
| CuO | Nanofibers | NO2 | 500–850 | 25 | 0.5 | 391 | 66 | 110 | 0.05 | [54] |
| Co3O4 | Nanosheets | CO | 200–400 | 100 | 5 | 2.4 | 14 | 36 | - | [63] |
| Materials | Morphology | Target Gas | D (nm) | OOT (°C) | Con (ppm) | Response (Ra/Rg) | Response Time (s) | Recovery Time (s) | LOD (ppm) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt-Fe2O3 | Nanowires | H2S | 100 | 175 | 10 | 157 | - | - | 1 | [67] |
| Pt-In2O3 | Nanofibers | NO2 | 90 | 25 | 1 | 23.9 (Rg/Ra) | - | 358 | 0.01 | [68] |
| Pt-SnO2 | Nanoparticles | C2H5OH | - | 350 | 2 | 93.56 | 9.2 | - | 0.1 | [69] |
| PdO-SnO2 | Nanotubes | NO2 | 600 | 30 | 100 | 20.30 | 1.33 | 22.6 | 0.01 | [70] |
| Pd-CeO2 | Nanofibers | CH3OH | 80 | 200 | 100 | 6.95 | 5 | 1 | 5 | [71] |
| Ag-WO3 | Nanofibers | NO2 | 103 ± 9 | 225 | 5 | 90.3 (Rg/Ra) | 714 | 522 | 0.5 | [72] |
| Ag-Fe2O3 | Nanofibers | H2S | 43 | 30 | 100 | 19.43 | - | - | 3 | [73] |
| GNP-TiO2 | Nanofibers | CO | 100 | 250 | 0.03 | 75 | 3 | 4 | 0.0007 | [74] |
| Au-In-ZnSnO3 | Nanofibers | C3H6O | 300 | 200 | 50 | 19.3 | 10 | 13 | 10 | [75] |
| Rh-ZnO | Nanofibers | NO2 | 200 | 150 | 10 | 36.17 (Rg/Ra) | 32 | 512 | 0.05 | [81] |
| Rh-SnO2 | Nanofibers | C3H6O | 150 | 200 | 50 | 60.6 | - | - | 1 | [90] |
| Ni/Au-In2O3 | Nanotubes | C2H5OH | 80–100 | 180 | 50 | 175 | 8 | 265 | 1 | [91] |
| PdO/NiO-In2O3 | Nanotubes | H2 | 200 | 160 | 5 | 487.52 | 1 | 336 | 0.1 | [92] |
| PtO2-SnO2 | Nanofibers | C3H6O | 150–250 | 400 | 5 | 194.15 | - | - | - | [93] |
| Pd@ZnO-WO3 | Nanofibers | C7H8 | 400–850 | 350 | 100 | 4.37 | 20 | - | - | [82] |
| Pt-WO3 | Nanofiber | C3H6O | 450 | 350 | 5 | 88.04 ± 3.18 | - | - | 0.4 | [87] |
| Pt-SnO2 | Nanotubes | H2S | 500 | 350 | 5 | 93.55 | - | - | - | [88] |
| Pt-WO3 | Nanofibers | NO2 | 887.2 | 350 | 5 | 834.2 ± 20.1 | - | - | 0.1 | [89] |
| Materials | Morphology | Target Gas | D (nm) | OOT (°C) | Con (ppm) | Response (Ra/Rg) | Response Time (s) | Recovery Time (s) | LOD (ppm) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ni-In2O3 | Nanotubes | C2H5OH | 100 | 220 | 100 | 49.74 | 3 | 49 | 5 | [95] |
| W-CeO2 | Nanofibers | C2H5OH | 130 | 200 | 100 | 10.2 | 7.04 | 55.30 | 1 | [96] |
| Yb-In2O3 | Nanofibers | C2H5OH | 75 ± 5 | 25 | 10 | 40 | - | - | 1 | [101] |
| Pr-BiFeO3 | Nanofibers | CH2O | 143.3 | 190 | 50 | 17.6 (Rg/Ra) | 17 | 19 | 5 | [104] |
| Eu-SnO2 | Nanofibers | C3H6O | 103 | 280 | 100 | 33.2 | 4 | 3 | 0.3 | [106] |
| K-CdGa2O4 | Nanofibers | CH2O | 75 | 120 | 10 | 90 | 1 | 62 | 0.02 | [107] |
| Ca-Fe2O3 Ca-Fe2O3 | Nanotubes Nanotubes | C2H5OH | 60 | 200 | 100 | 26.8 | - | - | 5 | [108] |
| C3H6O | 60 | 200 | 100 | 24.9 | - | - | 5 | [108] | ||
| Ca-In2O3 | Nanotubes | C2H5OH | 80 | 240 | 100 | 83.3 | 2 | 56 | 5 | [113] |
| Materials | Morphology | Target Gas | D (nm) | OOT (°C) | Con (ppm) | Response (Ra/Rg) | Response Time (s) | Recovery Time (s) | LOD (ppm) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| In2O3–ZnO | Porous nanofibers | C7H8 | 250 | 30 | 100 | 14.63 | 14 | 201 | 1 | [115] |
| SnO2/ZnO | Nanomaterials | C2H5OH | 191.7 | 300 | 100 | 31.6 | 10 | 56 | 5 | [118] |
| SnO2/ZnO | Nanomaterials | C3H6O | 191.7 | 300 | 100 | 22.7 | 5 | 10 | 5 | [118] |
| SnO2/Ga2O3 | Nanomaterials | C2H5OH | 136.5 | 300 | 100 | 33 | 2 | 37 | 5 | [118] |
| SnO2/WO3 | Nanomaterials | C8H10 | 55.7 | 300 | 100 | 9.8 | 12 | 12 | 5 | [118] |
| rGO/α-Fe2O3 | Nanocomposites | C3H6O | 100 | 375 | 100 | 8.9 | 3 | 9 | 5 | [125] |
| RGO-CuO | Nanofibers | H2S | 50 | 300 | 10 | 11.7 (Rg/Ra) | - | - | 1 | [126] |
| rGO-NiO | Nanofibers | NH3 | 230–350 | 27 | 50 | - | 32 | 38 | 50 | [10] |
| WO3-SnO2 | Core-shell nanofibers | C2H5OH | 100 | 220 | 10 | 5.09 | 18.5 | 282 | - | [132] |
| C-N/SnO2/D-ZnO/Au | Nanoparticles | C6H15N | 500–600 | 80 | 50 | 1970 | 18 | 6 | - | [133] |
| SnO2/ZnO | Nanoparticles | C2H5OH | 160–180 | 260 | 100 | 366 | 8 | 45 | - | [134] |
| In2O3-SnO2 | Core-shell nanocomposite | HCHO | 153 | 120 | 100 | 180.1 | 3 | 3.6 | 0.01 | [135] |
| CuZnFe2O4 | Nanofibers | H2 | 18 ± 9 | 250 | 500 | - | 6.5 | - | 50 | [155] |
| CuO-Fe2O3 | Nanofibers | NO2 | 50–100 | 300 | 10 | 15.3 | - | - | 1 | [141] |
| CuO-Fe2O3 | Nanofibers | H2S | 50–100 | 350 | 1 | 10 | - | - | 0.1 | [141] |
| NiFe2O4 | Nanofibers | H2S | 70–80 | 350 | 1 | 4.31 | 11 | 278 | 0.1 | [143] |
| NiFe2O4 | Nanofibers | NO2 | 70–80 | 350 | 10 | 4.25 | 27 | 303 | 1 | [143] |
| PrFeO3 | Nanofibers | C3H6O | 130 | 180 | 500 | 234.4 | 7 | 6 | 10 | [153] |
| ErFeO3 | Nanofibers | C2H5OH | 250 | 230 | 100 | 15.8 (Rg/Ra) | 61 | 39 | 0.035 | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Chen, H.; Chen, J.; Song, M. Gas Sensors Based on Semiconductor Metal Oxides Fabricated by Electrospinning: A Review. Sensors 2024, 24, 2962. https://doi.org/10.3390/s24102962
Chen H, Chen H, Chen J, Song M. Gas Sensors Based on Semiconductor Metal Oxides Fabricated by Electrospinning: A Review. Sensors. 2024; 24(10):2962. https://doi.org/10.3390/s24102962
Chicago/Turabian StyleChen, Hao, Huayang Chen, Jiabao Chen, and Mingxin Song. 2024. "Gas Sensors Based on Semiconductor Metal Oxides Fabricated by Electrospinning: A Review" Sensors 24, no. 10: 2962. https://doi.org/10.3390/s24102962





