Experimental Studies of Fluidized Bed Calcination of Granulated Clay Material
Abstract
:1. Introduction
Methods of Calcination of Clay Raw Materials
2. Research Methodology
2.1. Determination of the Degree of Calcination
- —the degree of calcination of the material after calcination;
- —the loss on ignition after calcination;
- —the loss on ignition of the raw clay material.
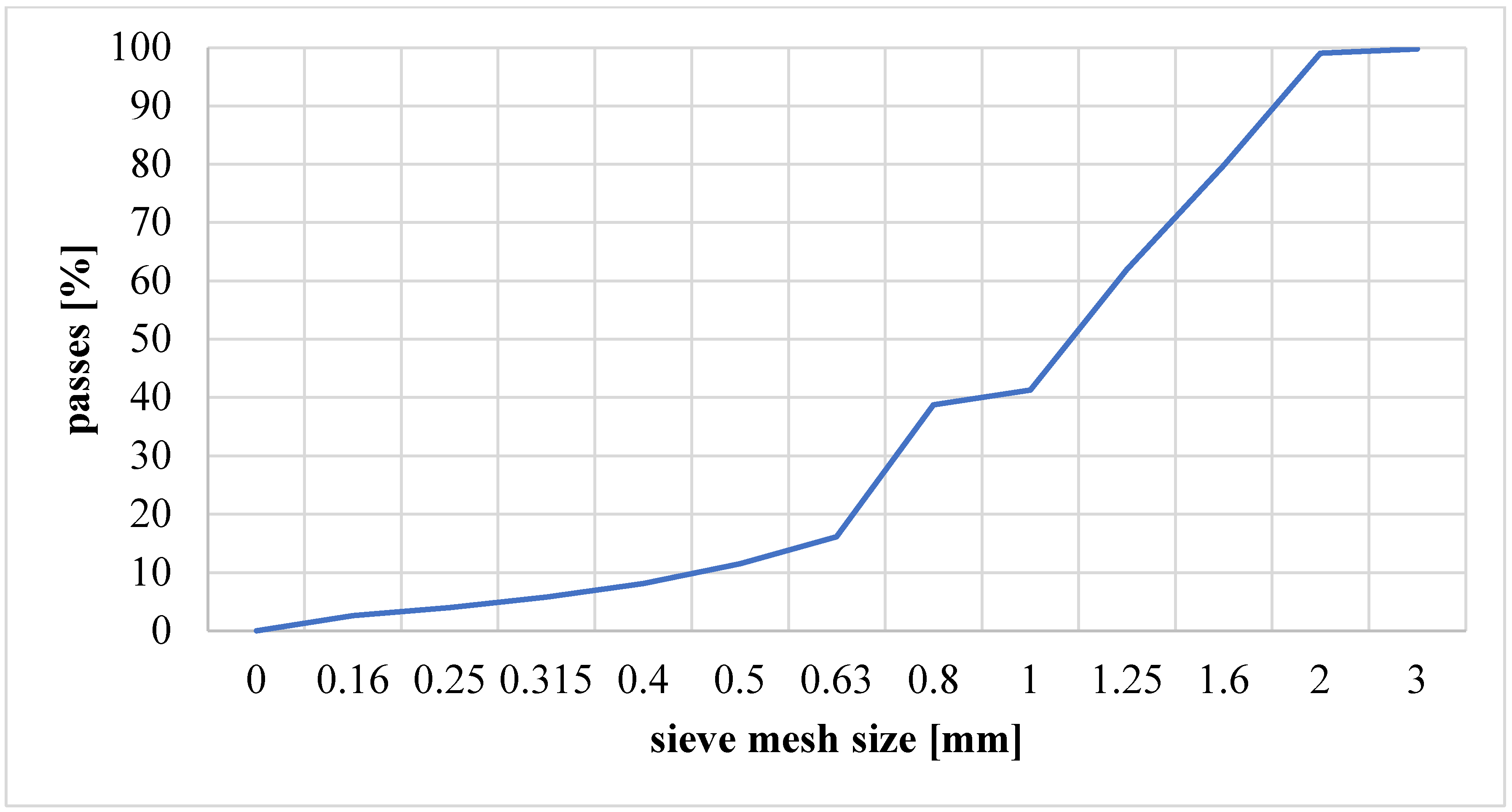
2.2. Characteristics of the Research Material
2.3. Fluidization Parameters of Clay Material
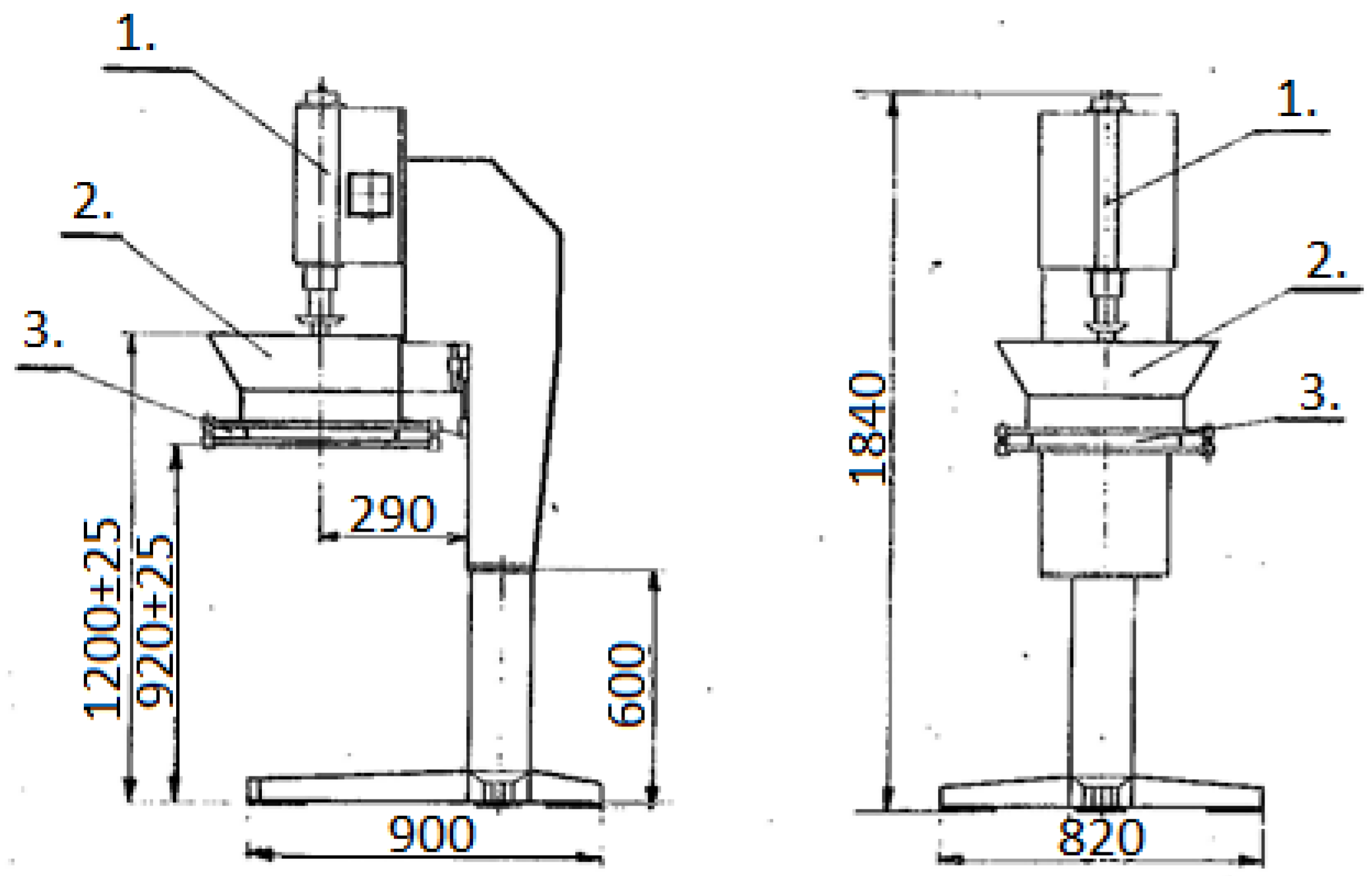
2.4. Experimental Stand
3. Experimental Research on Feeding a Fluidized Bed Reactor with Carbon Fuels
3.1. Fuel Characteristics
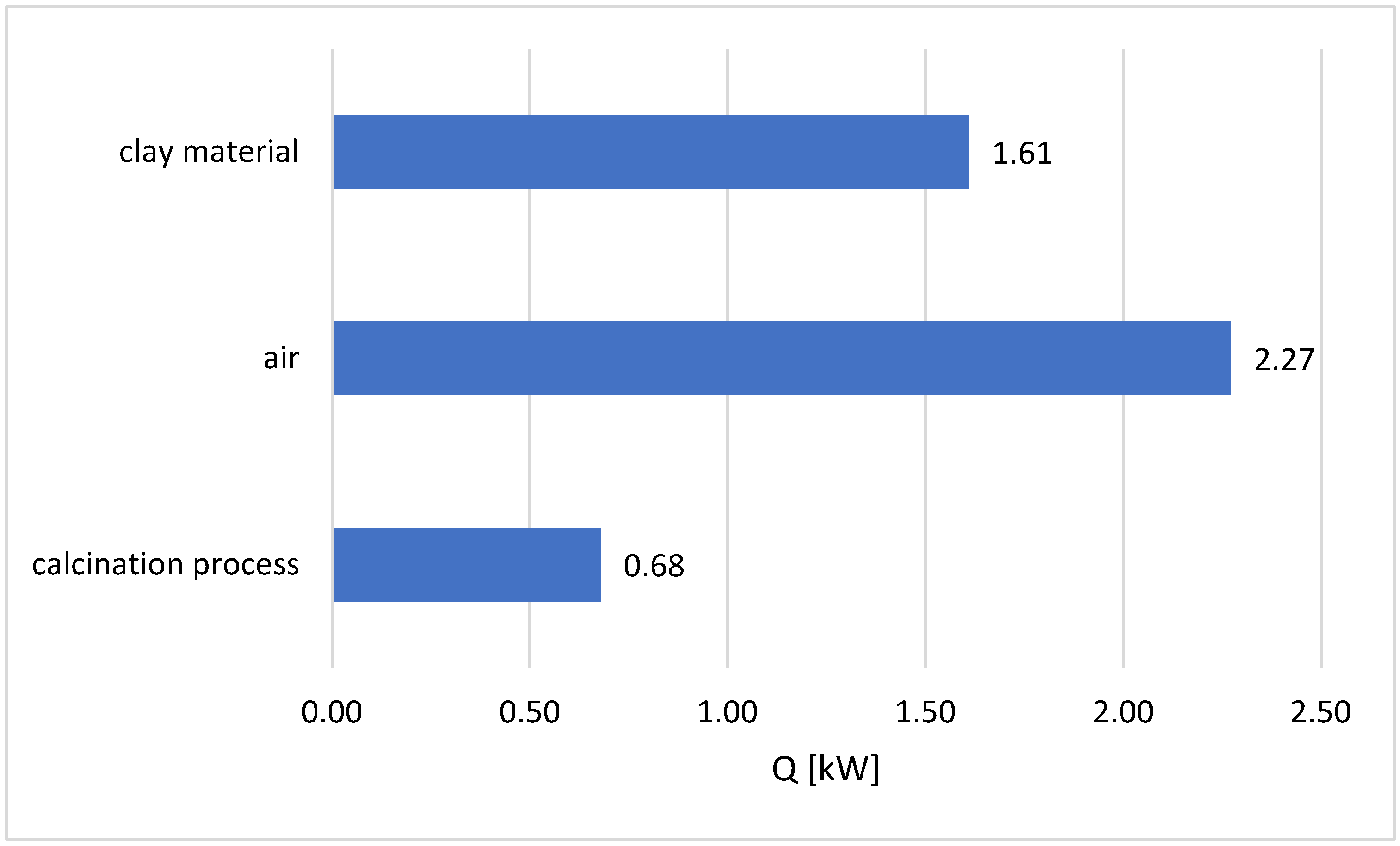
3.2. Heat Flow Demand for Calcination
- For the process of heating the total moisture of the clay material, evaporation, superheating of water vapour and to heat the clay material;
- To heat the air;
- For the calcination process.
3.3. Results of Experimental Studies
4. Discussion
5. Conclusions
- (1)
- The granulation process of clay material facilitates in obtaining material with the desired quantitative and qualitative parameters. Thanks to this, it is possible to obtain a final product with various properties required by their recipients.
- (2)
- Coal fuels can be successfully used in the fluidized bed calcination of clay materials.
- (3)
- When using solid fuels for the fluidized bed calcination process, the particle distribution of the fuels is crucial. The use of coal particles <0.75 mm in the tested fluidized bed calcination process resulted in their blowing out of the reactor chamber, increasing the energy deficit of the process and increasing the loss of incomplete combustion. The use of coal particles >1.6 mm in the fluidized calcination process resulted in insufficient time for their complete combustion. As a result, there was a carbon residue in the final product, which may have disqualified the product.
- (4)
- When anthracite is used as the basic fuel in the fluidized bed thermal calcination process, the control of the bed temperature is a very important factor. At a temperature below the fuel ignition temperature [39], tar may be released on the anthracite surface. As a consequence, this leads to the fusion of clay material particles with fuel particles and the formation of agglomerates or sintered particles, which, in the event of their rapid accumulation, may lead to a deterioration of the fluidization process and even to the defluidization of the bed.
- (5)
- Research on the calcination process using hard coal type 31.2 showed that cheaper and more easily available energetic hard coal can be used. When hard coal was used, neither the release of tar nor the formation of agglomerates was observed.
- (6)
- The particle analysis showed that the clay granulate is fragmented during the fluidized bed calcination process. The first reason is the process of the abrasion of its external surface as a result of the mutual interaction of the particles of clay material granules, and the second is the mechanical impact and the rapid occurrence of dehydration and dehydroxylation processes, which consequently leads to the disintegration of granulate particles.
- (7)
- The analysis showed that despite the smallest fraction of clay granules being blown out of the calcination chamber (particles <1354 µm), these particles are also calcined. The degree of their calcination is very high and amounts to >99%. In the future, the use of the appropriately selected separators may not only reduce the losses of the smallest fraction of particles during the fluidized bed calcination process but may also be used to segregate material particles according to particle size.
- (8)
- The balance calculations presented in the work indicate that almost 50% of the total heat demand in the calcination process is used to heat the air necessary for the fluidization of the bed—Figure 6. The use of lower fluidization velocities for a range of particles smaller than that assumed in the study will result in a significant reduction in energy expenditure on the calcination process.
- (9)
- Due to the high temperature and volume of reaction gases leaving the calcination reactor, it is advisable to use heat exchangers to reduce the moisture of the raw clay.
- (10)
- The positive results of tests on the fluidized bed calcination process using fuels such as anthracite and thermal hard coal indicate purposeful research into the possibility of using other solid fuels in the fluidized bed calcination process, i.e., biomass fuels and other alternative fuels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratajczak, T.; Hycnar, E.; Bożęcki, P. Kryterium mineralogiczne jako element oceny przydatności niektórych polskich surowców ilastych do budowy przesłon hydroizolacyjnych. Górnictwo Odkryw. Inst. Górnictwa Odkryw. Poltegor-Inst. 2016, 57, 19–25. [Google Scholar]
- Stoch, L. Minerały Ilaste; Wydawnictwa Geologiczne: Warsaw, Poland, 1974. [Google Scholar]
- Ito, A.; Wagai, R. Global distribution of clay-size minerals on land surface for biogeochemical and climatological studies. Sci. Data 2017, 4, 170103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Liu, X.W.; Hu, Y.H. Investigation of the thermal behaviour and decomposition kinetics of kaolinite. Clay Miner. 2015, 50, 199–209. [Google Scholar] [CrossRef]
- Hanein, T.; Thienel, K.C.; Zunino, F.; Marsh, A.T.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.; Ben Haha, M.; Avet, F.; et al. Clay calcination technology: State-of-the-art review by the RILEM TC 282-CCL. Mater. Struct. 2022, 55, 3. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Recent progress of utilization of activated kaolinitic clay in cementitious construction materials. Compos. Part B Eng. 2021, 211, 108636. [Google Scholar] [CrossRef]
- Nadeem, A.; Memon, S.A.; Lo, T.Y. Mechanical performance, durability, qualitative and quantitative analysis of microstructure of fly ash and metakaolin mortar at elevated temperatures. Constr. Build. Mater. 2013, 38, 338–347. [Google Scholar] [CrossRef]
- Madandoust, R.; Mousavi, S.Y. Fresh and hardened properties of self-compacting concrete containing metakaolin. Constr. Build. Mater. 2012, 35, 752–760. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Małaszkiewicz, D. Metakaolinit jako pucolanowy dodatek do betonu—Przegląd stanu wiedzy. Bud. I Inżynieria Sr. 2015, 6, 81–94. [Google Scholar]
- Ilić, B.R.; Mitrović, A.A.; Miličić, L.R. Thermal treatment of kaolin clay to obtain metakaolin. Hem. Ind. 2010, 64, 351–356. [Google Scholar] [CrossRef]
- Cassagnabère, F.; Escadeillas, G.; Mouret, M. Study of the reactivity of cement/metakaolin binders at early age for specific use in steam cured precast concrete. Constr. Build. Mater. 2009, 23, 775–784. [Google Scholar] [CrossRef]
- Neville, A.M. Właściwości Betonu; Polski Cement: Kraków, Poland, 2012; pp. 90–93. [Google Scholar]
- De Belie, N.; Soutsos, M.; Gruyaert, E. Properties of Fresh and Hardened Concrete Containing Supplementary Cementitious Materials; RILEM TC 238-SCM StAR; Springer: Berlin, Germany, 2018; Volume 25. [Google Scholar]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Shah, V.; Parashar, A.; Mishra, G.; Medepalli, S.; Krishnan, S.; Bishnoi, S. Influence of cement replacement by limestone calcined clay pozzolan on the engineering properties of mortar and concrete. Adv. Cem. Res. 2020, 32, 101–111. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.M.C.; Lund, J.; Herfort, D. Influence of fineness of raw clay and calcination temperature on the performance of calcined clay-limestone blended cements. Appl. Clay Sci. 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Zunino, F.; Martirena Hernandez, F.; Scrivener, K. Limestone calcined clay cements (LC3). ACI Mater. J. 2021, 118, 49. [Google Scholar]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Pouhet, R.; Cyr, M. Formulation and performance of flash metakaolin geopolymer concretes. Constr. Build. Mater. 2016, 120, 150–160. [Google Scholar] [CrossRef]
- Boonjaeng, S.; Chindaprasirt, P.; Pimraksa, K. Lime-calcined clay materials with alkaline activation: Phase development and reaction transition zone. Appl. Clay Sci. 2014, 95, 357–364. [Google Scholar] [CrossRef]
- Ruan S et al Solidification of waste excavation clay using reactive magnesia, quicklime, sodium carbonate and early-age oven curing. Constr. Build. Mater. 2020, 258, 120333. [CrossRef]
- Piech, J. Piece ceramiczne i Szklarskie; AGH [Akademia Górniczo-Hutnicza] Uczelniane Wydawnictwa Naukowo-Dydaktyczne: Kraków, Poland, 2001; pp. 83–149. [Google Scholar]
- Teklay, A.; Yin, C.; Rosendahl, L.; Køhler, L.L. Experimental and modeling study of flash calcination of kaolinite rich clay particles in a gas suspension calciner. Appl. Clay Sci. 2015, 103, 10–19. [Google Scholar] [CrossRef]
- Gonzalez, R.S.; Flamant, G. Technical and economic feasibility analysis of using concentrated solar thermal technology in the cement production process: Hybrid approach—A case study. J. Sol. Energy Eng. 2014, 136, 2. [Google Scholar] [CrossRef]
- Abanades, S.; André, L. Design and demonstration of a high temperature solar-heated rotary tube reactor for continuous particles calcination. Appl. Energy 2018, 212, 1310–1320. [Google Scholar] [CrossRef]
- Makul, N.; Rattanadecho, P.; Agrawal, D.K. Applications of microwave energy in cement and concrete—A review. Renew. Sustain. Energy Rev. 2014, 37, 715–733. [Google Scholar] [CrossRef]
- Reinosa, J.J.; García-Baños, B.; Catalá-Civera, J.M.; Fernández, J.F. A step ahead on efficient microwave heating for kaolinite. Appl. Clay Sci. 2019, 168, 237–243. [Google Scholar] [CrossRef]
- Burman, T.; Engvall, J. Evaluation of Usage of Plasma Torches in Cement Production. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2019. [Google Scholar]
- Kurdowski, W. Kilka uwag o piecach wapienniczych. Cem. Wapno Beton 2004, 9, 33–45. [Google Scholar]
- Khadilkar, A.; Rozelle, P.L.; Pisupati, S.V. Models of agglomerate growth in fluidized bed reactors: Critical review, status and applications. Powder Technol. 2014, 264, 216–228. [Google Scholar] [CrossRef]
- Scala, F.; Salatino, P. Dolomite attrition during fluidized-bed calcination and sulfation. Combust. Sci. Technol. 2003, 175, 2201–2216. [Google Scholar] [CrossRef]
- Zhang, W. A Review of Techniques for the Process Intensification of Fluidized Bed Reactors. Chin. J. Chem. Eng. 2009, 17, 688–702. [Google Scholar] [CrossRef]
- Shuai, Y.; Yuexin, H.; Yanjun, L.; Peng, G.; Jianwen, Y. Effect of calcination temperature on activation behaviors of coal-series kaolin by fluidized bed calcination. Physicochem. Probl. Miner. Process. 2018, 54, 590–600. [Google Scholar] [CrossRef]
- Kaczyńska, K.; Kaczyński, K.; Pełka, P. Calcination of Clay Raw Materials in a Fluidized Bed. Materials 2021, 14, 3989. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.S.; Rao, A.V.R. Production of clay pozzolana by fluidized bed technique. Trans. Indian Ceram. Soc. 1978, 37, 165–171. [Google Scholar] [CrossRef]
- Emmanuel, A.C.; Haldar, P.; Maity, S.; Bishnoi, S. Second pilot production of limestone calcined clay cement in India: The experience. Indian Concr. J. 2016, 90, 57–64. [Google Scholar]
- Prins, W.; Siemons, R.; Van Swaaij, W.P.M.; Radovanovic, M. Devolatilization and ignition of coal particles in a two-dimensional fluidized bed. Combust. Flame 1989, 75, 57–79. [Google Scholar] [CrossRef]
- Wójcicki, S. Spalanie; Wydaw. Nauk.-Techn.: Warsaw, Poland, 1969; pp. 375–397. [Google Scholar]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Wyrwicki, R. Analiza Deriwatograficzna Skał Ilastych; Wydawnictwo Uniwersytetu Warszawskiego: Warsaw, Poland, 1998. [Google Scholar]
- Internal Report CEWAP sp. z o.o. February 2017.
- PNG-04511:1980; Polish Standard: Solid Fuels. Determination of Moisture Content. Polish Committee for Standardization: Warsaw, Poland, 1980.
- PN-81/G-04516; Polish Standard: Solid Fuels. Determination of Volatile Matter Content. Polish Committee for Standardization: Warsaw, Poland, 1981.
- PN-G-04512:1980; Polish Standard: Solid Fuels. Determination of Ash by Weight. Polish Committee for Standardization: Warsaw, Poland, 1980.
- PN EN 1097-3: 2000; Tests for Mechanical and Physical Properties of Aggregates—Part 3: Determination of Loose Bulk Density and Voids. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Basu, P.; Fraser, S.A. Circulating Fluidized Bed Boilers, Design and Operations; Butterworth-Heinemann: Boston, MA, USA, 1991. [Google Scholar]
- Geldart, D. Types of Gas Fluidization. Powder Technol. 1973, 7, 285–291. [Google Scholar] [CrossRef]
- Grammelis, P.; Margaritis, N.; Karampinis, E. 2—Solid fuel types for energy generation: Coal and fossil carbon-derivative solid fuels. In Fuel Flexible Energy Generation; Oakey, J., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 29–58. [Google Scholar] [CrossRef]









| Compound | Content, % |
|---|---|
| SiO2 | 58.50% |
| Al2O3 | 32.70% |
| Fe2O3 | 2.51% |
| TiO2 | 2.01% |
| CaO | 0.33% |
| MgO | 0.61% |
| K2O | 2.99% |
| Na2O | 0.11% |
| other | 0.24% |
| together | 100% |
| Parameter | Moisture [%] | Volatile Matter Content [%] | Clay Content [%] |
|---|---|---|---|
| value | 4.32 | 1.01 | 94.67 |
| T [°C] | 27 | 850 |
|---|---|---|
| Ar [−] | 67,645.65 | 3097.19 |
| Remf [−] | 31.96 | 2.23 |
| Umf [m/s] | 0.41 | 0.25 |
| Ut [m/s] | 7.35 | 0.63 |
| Utr [m/s] | 5.39 | 0.81 |
| Umb [m/s] | 0.41 | 0.25 |
| Technical Analysis | Anthracite 1 | Hard Coal Type 31.2 (Flaming) | Anthracite 2 |
|---|---|---|---|
| Moisture content [%] | 2.97 | 8.12 | 4.8 |
| Volatile content [%] | 9.43 | 30.30 | 10.07 |
| Fixed carbon [%] | 77.38 | 47.20 | 82.68 |
| Ash content [%] | 10.22 | 14.38 | 2.45 |
| Heat of combustion [MJ/kg] | 29.28 | 26.10 | 31.54 |
| Calorific value [MJ/kg] | 28.27 | 25.00 | 31.12 |
| Fuel | Nitrogen, N [%] | Carbon, C [%] | Hydrogen, H [%] | Sulphur, S [%] |
|---|---|---|---|---|
| Anthracite 1 | 0.86 | 62.45 | 4.31 | 0.85 |
| Hard coal type 31.2 (flaming) | 0.87 | 55.81 | 4.45 | 1.44 |
| Anthracite 2 | 0.44 | 76.45 | 1.40 | 0.33 |
| No. | Type of Fuel/Type of Coal | Fluidization Velocity [m/s] | Turning Off the Air Heater | Loss on Ignition [%] | Degree of Calcination [%] |
|---|---|---|---|---|---|
| 1 | Electric power supply | 0.26 | - | 0.22 | 97.92 |
| 2 | 0.26 | - | 0.01 | 99.91 | |
| 3 | Anthracite 1 | 0.28 | NO | not marked | |
| 4 | 0.28 | NO | 0.24 | 97.73 | |
| 5 | 0.28 | YES | 0.45 | 95.74 | |
| 6 | 0.28 | NO | 0.51 | 95.18 | |
| 7 | 0.28 | YES | 0.66 | 93.76 | |
| 8 | Hard coal type 31.2 | 0.29 | NO | 0.38 | 96.41 |
| 9 | 0.28 | YES | 0.01 | 99.91 | |
| 10 | 0.28 | NO | 0.06 | 99.43 | |
| 11 | Anthracite 2 | 0.29 | NO | 6.73 | 36.34 |
| 12 | 0.28 | NO | 0.66 | 93.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczyńska, K.; Pełka, P. Experimental Studies of Fluidized Bed Calcination of Granulated Clay Material. Materials 2024, 17, 2185. https://doi.org/10.3390/ma17102185
Kaczyńska K, Pełka P. Experimental Studies of Fluidized Bed Calcination of Granulated Clay Material. Materials. 2024; 17(10):2185. https://doi.org/10.3390/ma17102185
Chicago/Turabian StyleKaczyńska, Katarzyna, and Piotr Pełka. 2024. "Experimental Studies of Fluidized Bed Calcination of Granulated Clay Material" Materials 17, no. 10: 2185. https://doi.org/10.3390/ma17102185





