Waste Glass Upcycling Supported by Alkali Activation: An Overview
Abstract
:1. Introduction
2. Overview of Waste Glass Used in Alkali Activation
2.1. Soda-Lime Glasses
2.2. Borosilicate Glasses
2.3. Aluminosilicate Glasses
2.4. Lead Silicate Glass
2.5. Glass Wool and Rock Wool
2.6. Volcanic Glass
2.7. Waste-Derived Glasses
| Glass Type | Applications | Approximate Composition | Description | Ref. |
|---|---|---|---|---|
| Soda-lime | Container glass Window panes Building sector | O 11% CaO 1% MgO | Prepared by heating a mixture that includes limestone (), silica sand (), soda ash (), and a few additional ingredients. Crushed jars, bottles, containers, discarded windows and doors, and scraps from glass unit production are typical sources of soda-lime glass waste. | [33] |
| Borosilicate | Pharmaceutical glass Laboratory glassware Optical glass | 70–80% O | Typically made up of silicon dioxide, boron trioxide, aluminium oxide, and a few alkaline earth oxides. Due to its low alkali content, this glass offers excellent chemical durability and thermal shock resistance. It finds widespread use as laboratory equipment and pharmaceutical storage containers in the chemistry sector. | [72] |
| Aluminosilicate | Fibre glass Mobile phone screens Combustion tubes | 23% CaO | Common uses include insulation, fibre optic cables, strengthened parts, and smartphone displays. | [73] |
| Lead silicate | TV screens (CRT) Artistic ware Absorption of X-rays | 55–65% 25–30% PbO 12–16% O 13–15% O | Often just called “crystal” for short. It is used often in ceramic glazes, cathode ray tubes (CRTs), and other artistic glassware. PbO is present in large quantities, which gives the product its shiny appearance. | [74] |
| Glass wool | Pipe insulation Suspended ceilings Higher-temperature insulation | 8% CaO O 3% MgO 1–2% | Construction and demolition (C&D) waste and mineral wool production are two major sources of glass wool waste. The majority of this mineral waste is unutilised at the moment. | [75] |
| Volcanic glass | - | 9.8% CaO 6.3% MgO O O | Easily accessible and inexpensive, volcanic ash (VA) is a substance with many potential applications. Due of its chemical composition and amorphous atomic structure, volcanic ash has been proposed as a replacement for Portland cement and other alkali-activated building materials. | [76] |
3. Alkali Activation Mechanism in Waste Glass
3.1. Concentration of Alkaline Solutions
3.2. Pre-Curing Time
4. Properties of AAMs Incorporating Glass Waste as a Raw Material
4.1. Workability
4.2. Setting Time
4.3. Density
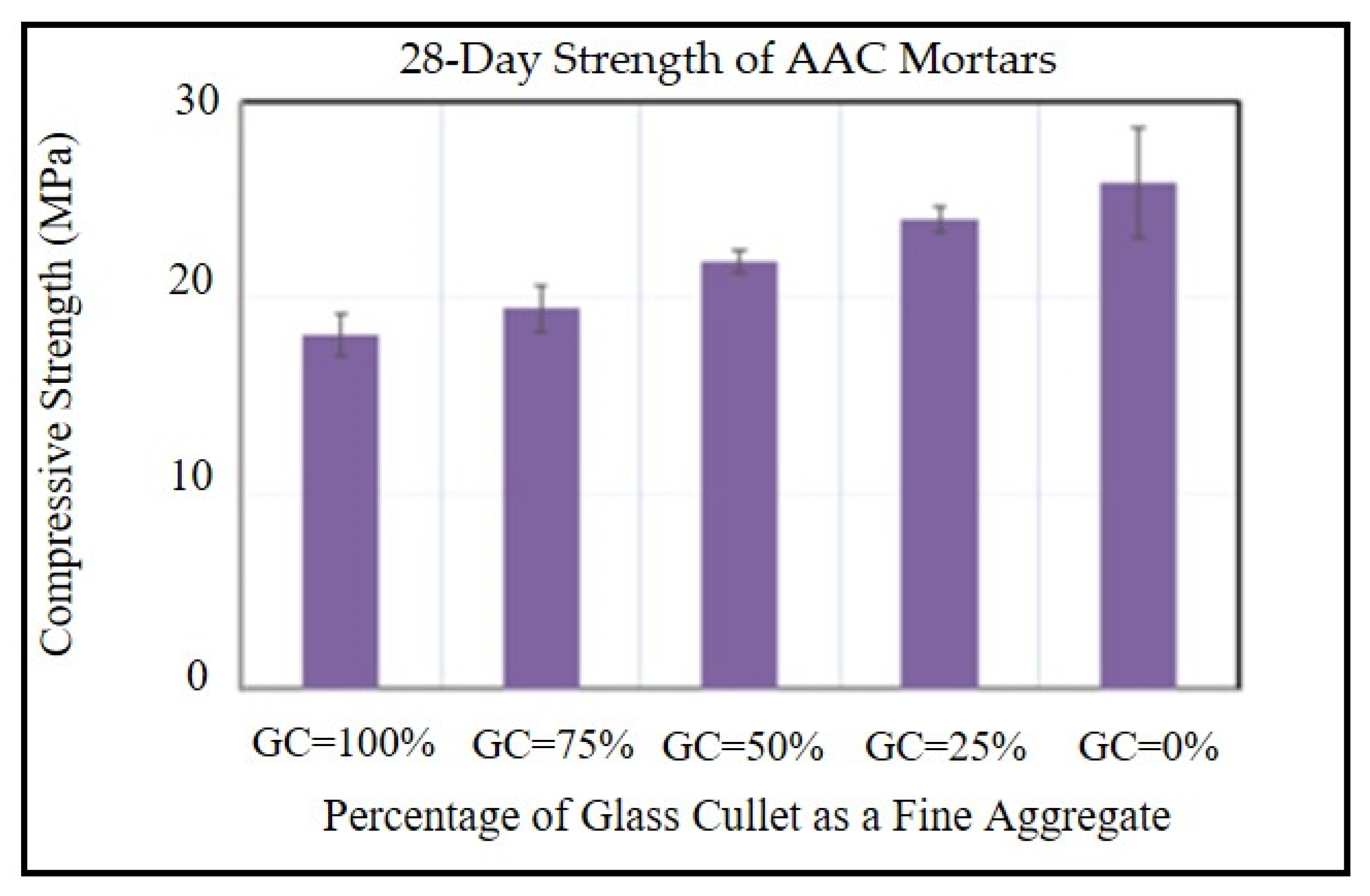
4.4. Compressive Strength
5. Applications of Waste-Glass-Based Alkali-Activated Materials
5.1. Soil Stabilisation
5.2. Road-Base Stabilisation and Tile Production
5.3. Geopolymer Concrete
5.4. Thermal and Acoustic Insulation
5.5. Other Applications
6. Conclusions
- Waste glasses from various sources may be used to produce AAMs. The reactivity of glasses was significantly impacted by the various oxide amounts in waste glasses and, subsequently, the performance of alkali-activated materials and hydration products.
- Using different types of glass results in different hydration products for AAMs. Alkali-activated low-calcium waste glass precursors mainly produce NA-S-(H) gels, while alkali-activated high calcium materials primarily produce C-(A)-S-H gels.
- Similar levels of success have been seen with the utilisation of processed glass waste in foam glass as with its denser counterpart. Glass cullet is a valuable resource due to its low weight, excellent thermal and chemical durability, and solid insulating qualities, which may be utilised in a range of applications. However, the foaming process’s efficiency also relies on several intrinsic characteristics of the glass employed, including the kind of glass, its fineness, and its reactivity, in addition to the foaming agent and sintering temperatures and timeframes.
- Lightweight inorganic polymers may be produced by activating NaOH solutions with low molarities; the ideal concentration range for the alkali activator is between 2 and 8 M, depending on the kind of activator and the mixture percentage. NaOH and KOH are the alkali activators often used for the alkali activation of waste glass. The activation duration, pre-curing conditions, activator concentration, and activator type may also influence the features of the finished product.
- With applications ranging from construction to fireproof safety, carrier media in bioreactors, adsorbents, energy storage, medications, the solidification/stabilisation of contaminants, pH buffers, and radioactive waste containers, alkali-activated materials (AAM) have a lot of potential as versatile materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nodehi, M.; Ozbakkaloglu, T.; Gholampour, A.; Mohammed, T.; Shi, X. The effect of curing regimes on physico-mechanical, microstructural and durability properties of alkali-activated materials: A review. Constr. Build. Mater. 2022, 321, 126335. [Google Scholar] [CrossRef]
- Qian, L.-P.; Xu, L.-Y.; Alrefaei, Y.; Wang, T.; Ishida, T.; Dai, J.-G. Artificial alkali-activated aggregates developed from wastes and by-products: A state-of-the-art review. Resour. Conserv. Recycl. 2022, 177, 105971. [Google Scholar] [CrossRef]
- Zakka, W.P.; Abdul Shukor Lim, N.H.; Chau Khun, M. A scientometric review of geopolymer concrete. J. Clean. Prod. 2021, 280, 124353. [Google Scholar] [CrossRef]
- Rincon Romero, A.; Salvo, M.; Bernardo, E. Up-cycling of vitrified bottom ash from MSWI into glass-ceramic foams by means of ‘inorganic gel casting’ and sinter-crystallization. Constr. Build. Mater. 2018, 192, 133–140. [Google Scholar] [CrossRef]
- Sun, B.; Ye, G.; De Schutter, G. A review: Reaction mechanism and strength of slag and fly ash-based alkali-activated materials. Constr. Build. Mater. 2022, 326, 126843. [Google Scholar] [CrossRef]
- Bucher, R.; Cyr, M.; Escadeillas, G. Performance-based evaluation of flash-metakaolin as cement replacement in marine structures—Case of chloride migration and corrosion. Constr. Build. Mater. 2021, 267, 120926. [Google Scholar] [CrossRef]
- Fermeglia, M.; Bevilacqua, P.; Cafaro, C.; Ceci, P.; Fardelli, A. Legal Pathways to Coal Phase-Out in Italy in 2025. Energies 2020, 13, 5605. [Google Scholar] [CrossRef]
- Lieberman, R.N.; Knop, Y.; Querol, X.; Moreno, N.; Muñoz-Quirós, C.; Mastai, Y.; Anker, Y.; Cohen, H. Environmental impact and potential use of coal fly ash and sub-economical quarry fine aggregates in concrete. J. Hazard. Mater. 2018, 344, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Panesar, D.K.; Kanraj, D.; Abualrous, Y. Effect of transportation of fly ash: Life cycle assessment and life cycle cost analysis of concrete. Cem. Concr. Compos. 2019, 99, 214–224. [Google Scholar] [CrossRef]
- Robert, D.; Baez, E.; Setunge, S. A new technology of transforming recycled glass waste to construction components. Constr. Build. Mater. 2021, 313, 125539. [Google Scholar] [CrossRef]
- Imtiaz, L.; Rehman, S.K.U.; Ali Memon, S.; Khizar Khan, M.; Faisal Javed, M. A Review of Recent Developments and Advances in Eco-Friendly Geopolymer Concrete. Appl. Sci 2020, 10, 7838. [Google Scholar] [CrossRef]
- Close the Glass Loop Collection Statistics 2019 Published on the Close the Glass Loop Website. Available online: https://www.closetheglassloop.eu (accessed on 12 January 2024).
- Yao, Z.; Ling, T.-C.; Sarker, P.K.; Su, W.; Liu, J.; Wu, W.; Tang, J. Recycling difficult-to-treat e-waste cathode-ray-tube glass as construction and building materials: A critical review. Renew. Sustain. Energy Rev. 2018, 81, 595–604. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Zhang, S. Dispersion, properties, and mechanisms of nanotechnology-modified alkali-activated materials: A review. Renew. Sustain. Energy Rev. 2024, 192, 114215. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S. Performance study and environmental evaluation of alkali-activated materials based on waste photovoltaic glass. J. Clean. Prod. 2022, 379, 134576. [Google Scholar] [CrossRef]
- Hujova, M.; Rabelo Monich, P.; Kankova, H.; Lucas, H.; Xakalashe, B.; Friedrich, B.; Kraxner, J.; Galusek, D.; Bernardo, E. New glass-based binders from engineered mixtures of inorganic waste. Int. J. Appl. Glas. Sci. 2021, 12, 570–580. [Google Scholar] [CrossRef]
- Grutzeck, M.; Kwan, S.; DiCola, M. Zeolite formation in alkali-activated cementitious systems. Cem. Concr. Res. 2004, 34, 949–955. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Pavoine, A. Investigations on the durability of alkali-activated recycled glass. Constr. Build. Mater. 2020, 236, 117477. [Google Scholar] [CrossRef]
- Zhang, B.; He, P.; Poon, C.S. Optimizing the use of recycled glass materials in alkali activated cement (AAC) based mortars. J. Clean. Prod. 2020, 255, 120228. [Google Scholar] [CrossRef]
- Ramteke, D.D.; Hujova, M.; Kraxner, J.; Galusek, D.; Romero, A.R.; Falcone, R.; Bernardo, E. Up-cycling of ‘unrecyclable’ glasses in glass-based foams by weak alkali-activation, gel casting and low-temperature sintering. J. Clean. Prod. 2021, 278, 123985. [Google Scholar] [CrossRef]
- Lago, D.; Kraxner, J.; Galusek, D.; Bernardo, E. Novel glass-based membranes for Cu adsorption: From alkali activation to sintering. Heliyon 2023, 9, e18221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Huang, B.; Zhou, H.; Ma, Y.; Jiang, X. A state-of-the-art review of crushed urban waste glass used in OPC and AAMs (geopolymer): Progress and challenges. Clean. Mater. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, Y.; Jiang, X.; Polaczyk, P.; Ma, Y.; Huang, B. Alkali-activated slag supplemented with waste glass powder: Laboratory characterization, thermodynamic modelling and sustainability analysis. J. Clean. Prod. 2021, 286, 125554. [Google Scholar] [CrossRef]
- McLellan, G.W.; Shand, E.B. Glass Engineering Handbook; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Lu, J.-X.; Poon, C.S. Use of waste glass in alkali activated cement mortar. Constr. Build. Mater. 2018, 160, 399–407. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N. An overview on the reuse of waste glasses in alkali-activated materials. Resour. Conserv. Recycl. 2019, 144, 297–309. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Roether, J.A.; Ercole, P.; Bernardo, E.; Boccaccini, A.R. Extensive reuse of soda-lime waste glass in fly ash-based geopolymers. Constr. Build. Mater. 2018, 188, 1077–1084. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; De Silva, V.R.S. Effect of soda-lime glass powder on alkali-activated binders: Rheology, strength and microstructure characterization. Constr. Build. Mater. 2020, 241, 118013. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Poinot, T. Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J. Mater. Sci. 2012, 47, 2782–2797. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass as a precursor in alkaline activation: Chemical process and hydration products. Constr. Build. Mater. 2017, 139, 342–354. [Google Scholar] [CrossRef]
- Rincón, A.; Giacomello, G.; Pasetto, M.; Bernardo, E. Novel ‘inorganic gel casting’ process for the manufacturing of glass foams. J. Eur. Ceram. Soc. 2017, 37, 2227–2234. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Rafferty, A.; Sajjia, M.; Olabi, A.G. Properties of Glass Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Smedskjaer, M.M.; Mauro, J.C.; Youngman, R.E.; Hogue, C.L.; Potuzak, M.; Yue, Y. Topological Principles of Borosilicate Glass Chemistry. J. Phys. Chem. B 2011, 115, 12930–12946. [Google Scholar] [CrossRef]
- Rincon Romero, A.; Tamburini, S.; Taveri, G.; Toušek, J.; Dlouhy, I.; Bernardo, E. Extension of the ‘Inorganic Gel Casting’ Process to the Manufacturing of Boro-Alumino-Silicate Glass Foams. Materials 2018, 11, 2545. [Google Scholar] [CrossRef]
- Tameni, G.; Cammelli, F.; Elsayed, H.; Stangherlin, F.; Bernardo, E. Upcycling of Boro-Alumino-Silicate Pharmaceutical Glass in Sustainable Construction Materials. Detritus 2022, 20, 17–21. [Google Scholar] [CrossRef]
- Mehta, A.; Karbouche, K.; Kraxner, J.; Elsayed, H.; Galusek, D.; Bernardo, E. Upcycling of Pharmaceutical Glass into Highly Porous Ceramics: From Foams to Membranes. Materials 2022, 15, 3784. [Google Scholar] [CrossRef]
- Cormier, L. Glasses: Aluminosilicates. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 496–518. [Google Scholar]
- Manikandan, P.; Natrayan, L.; Duraimurugan, S.; Vasugi, V. Influence of Waste Glass Powder as an Aluminosilicate Precursor in Synthesizing Ternary Blended Alkali-Activated Binder. Silicon 2022, 14, 7799–7808. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A.; Pham, T.M.; Yang, B. Glass fibre-reinforced polymer circular alkali-activated fly ash/slag concrete members under combined loading. Eng. Struct. 2019, 199, 109598. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio: Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernández-Jimenez, A.; Pena, P.; Palomo, A. Alkaline activation of synthetic aluminosilicate glass. Ceram. Int. 2014, 40, 5547–5558. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Palomo, A. Alternative prime materials for developing new cements: Alkaline activation of alkali aluminosilicate glasses. Ceram. Int. 2016, 42, 9333–9340. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Aparicio-Rebollo, E.; Fernández-Jimenez, A.; Palomo, A. Effect of calcium on the alkaline activation of aluminosilicate glass. Ceram. Int. 2016, 42, 7697–7707. [Google Scholar] [CrossRef]
- Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Cheeseman, C.R.; Payá, J. Alkali activation of vitreous calcium aluminosilicate derived from glass fiber waste. J. Sustain. Cem. Based Mater. 2012, 1, 83–93. [Google Scholar] [CrossRef]
- Rashidian-Dezfouli, H.; Rangaraju, P.R.; Kothala, V.S.K. Influence of selected parameters on compressive strength of geopolymer produced from ground glass fiber. Constr. Build. Mater. 2018, 162, 393–405. [Google Scholar] [CrossRef]
- Bauccio, M.A. International, ASM Engineered Materials Reference Book; ASM International: Materials Park, OH, USA, 1994. [Google Scholar]
- Angeli, F.; Jollivet, P.; Charpentier, T.; Fournier, M.; Gin, S. Structure and Chemical Durability of Lead Crystal Glass. Environ. Sci. Technol. 2016, 50, 11549–11558. [Google Scholar] [CrossRef]
- Long, W.-J.; Li, H.-D.; Ma, H.; Fang, Y.; Xing, F. Green alkali-activated mortar: Sustainable use of discarded cathode-ray tube glass powder as precursor. J. Clean. Prod. 2019, 229, 1082–1092. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Xie, R.; Li, X.; Cheng, J.; Yang, T. Performance of fly ash-based geopolymer mortars with waste cathode ray tubes glass fine aggregate: A comparative study with cement mortars. Constr. Build. Mater. 2022, 344, 128243. [Google Scholar] [CrossRef]
- Long, W.-J.; Zhang, X.; Xie, J.; Kou, S.; Luo, Q.; Wei, J.; Lin, C.; Feng, G.-L. Recycling of waste cathode ray tube glass through fly ash-slag geopolymer mortar. Constr. Build. Mater. 2022, 322, 126454. [Google Scholar] [CrossRef]
- Yliniemi, J.; Walkley, B.; Provis, J.L.; Kinnunen, P.; Illikainen, M. Nanostructural evolution of alkali-activated mineral wools. Cem. Concr. Compos. 2020, 106, 103472. [Google Scholar] [CrossRef]
- Yliniemi, J.; Kinnunen, P.; Karinkanta, P.; Illikainen, M. Utilization of Mineral Wools as Alkali-Activated Material Precursor. Materials 2016, 9, 312. [Google Scholar] [CrossRef]
- Väntsi, O.; Kärki, T. Mineral wool waste in Europe: A review of mineral wool waste quantity, quality, and current recycling methods. J. Mater. Cycles Waste Manag. 2014, 16, 62–72. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Adediran, A.; Yliniemi, J.; Luukkonen, T.; Illikainen, M. Effect of organic resin in glass wool waste and curing temperature on the synthesis and properties of alkali-activated pastes. Mater. Des. 2021, 212, 110287. [Google Scholar] [CrossRef]
- Pavlin, M.; Horvat, B.; Češnovar, M.; Ducman, V. The preparation and characterization of low-temperature foams based on the alkali activation of waste stone wool. Ceram. Int. 2022, 48, 17668–17681. [Google Scholar] [CrossRef]
- Mastali, M.; Zahra, A.; Hugo, K.; Faraz, R. Utilization of mineral wools in production of alkali activated materials. Constr. Build. Mater. 2021, 283, 122790. [Google Scholar] [CrossRef]
- Bernardo, E.; Elsayed, H.; Mazzi, A.; Tameni, G.; Gazzo, S.; Contrafatto, L. Double-life sustainable construction materials from alkali activation of volcanic ash/discarded glass mixture. Constr. Build. Mater. 2022, 359, 129540. [Google Scholar] [CrossRef]
- Barone, G.; Finocchiaro, C.; Lancellotti, I.; Leonelli, C.; Mazzoleni, P.; Sgarlata, C.; Stroscio, A. Potentiality of the Use of Pyroclastic Volcanic Residues in the Production of Alkali Activated Material. Waste Biomass Valorization 2021, 12, 1075–1094. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Mejía-Arcila, J.; Mejía de Gutiérrez, R.; Martínez, E. Life cycle assessment (LCA) of an alkali-activated binary concrete based on natural volcanic pozzolan: A comparative analysis to OPC concrete. Constr. Build. Mater. 2018, 176, 103–111. [Google Scholar] [CrossRef]
- Játiva, A.; Ruales, E.; Etxeberria, M. Volcanic Ash as a Sustainable Binder Material: An Extensive Review. Materials 2021, 14, 1302. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Churata, R.; Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Torres-Almirón, J.; Ortiz-Valdivia, Y.; Velasco, F. Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing. Buildings 2022, 12, 1118. [Google Scholar] [CrossRef]
- Firdous, R.; Stephan, D.; Djobo, J.N.Y. Natural pozzolan based geopolymers: A review on mechanical, microstructural and durability characteristics. Constr. Build. Mater. 2018, 190, 1251–1263. [Google Scholar] [CrossRef]
- Meyers, R.A. Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012. [Google Scholar]
- Miraki, H.; Shariatmadari, N.; Ghadir, P.; Jahandari, S.; Tao, Z.; Siddique, R. Clayey soil stabilization using alkali-activated volcanic ash and slag. J. Rock Mech. Geotech. Eng. 2022, 14, 576–591. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. 2—An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 19–47. [Google Scholar]
- Rincón, A.; Marangoni, M.; Cetin, S.; Bernardo, E. Recycling of inorganic waste in monolithic and cellular glass-based materials for structural and functional applications. J. Chem. Technol. Biotechnol. 2016, 91, 1946–1961. [Google Scholar] [CrossRef]
- Mehta, A.; Colusso, E.; Kraxner, J.; Galusek, D.; Bernardo, E. Waste-derived glass as a precursor for inorganic polymers: From foams to photocatalytic destructors for dye removal. Ceram. Int. 2022, 48 Pt A, 27631–27636. [Google Scholar] [CrossRef]
- Lee, J.-C.; Jang, B.-K.; Shon, C.-S.; Kim, J.-H.; Chung, C.-W. Potential use of borosilicate glass to make neutron shielding mortar: Enhancement of thermal neutron shielding and strength development and mitigation of alkali-silica reaction. J. Clean. Prod. 2019, 210, 638–645. [Google Scholar] [CrossRef]
- Mahmoud, M.; Kraxner, J.; Kaňková, H.; Hujová, M.; Chen, S.; Galusek, D.; Bernardo, E. Porous Glass Microspheres from Alkali-Activated Fiber Glass Waste. Materials 2022, 15, 1043. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Vieitez, E.; Eder, P.; Villanueva Krzyzaniak, A.; Saveyn, H. End-of-Waste Criteria for Glass Cullet: Technical Proposals; EUR 25220 EN; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Yliniemi, J.; Laitinen, O.; Kinnunen, P.; Illikainen, M. Pulverization of fibrous mineral wool waste. J. Mater. Cycles Waste Manag. 2017, 20, 1248–1256. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive strength and microstructure of assorted wastes incorporated geopolymer mortars: Effect of solution molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Kashani, A. Glass waste versus sand as aggregates: The characteristics of the evolving geopolymer binders. J. Clean. Prod. 2018, 193, 593–603. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass wastes for the development of geopolymer composites—Durability, thermal and microstructural properties: A review. Constr. Build. Mater. 2019, 222, 673–687. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Dissolution behaviour of source materials for synthesis of geopolymer binders: A kinetic approach. Int. J. Miner. Process. 2016, 153, 80–86. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Mamun, M.A.A.; Alyousef, R.; Ferdous, W. Waste Glass in Cement and Geopolymer Concretes: A Review on Durability and Challenges. Polymers 2021, 13, 2071. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- El-Naggar, M.R.; El-Dessouky, M.I. Re-use of waste glass in improving properties of metakaolin-based geopolymers: Mechanical and microstructure examinations. Constr. Build. Mater. 2017, 132, 543–555. [Google Scholar] [CrossRef]
- Monich, P.R.; Romero, A.R.; Höllen, D.; Bernardo, E. Porous glass-ceramics from alkali activation and sinter-crystallization of mixtures of waste glass and residues from plasma processing of municipal solid waste. J. Clean. Prod. 2018, 188, 871–878. [Google Scholar] [CrossRef]
- Rincón Romero, A.; Toniolo, N.; Boccaccini, A.R.; Bernardo, E. Glass-Ceramic Foams from ‘Weak Alkali Activation’ and Gel-Casting of Waste Glass/Fly Ash Mixtures. Materials 2019, 12, 588. [Google Scholar] [CrossRef]
- Pavlin, M.; Horvat, B.; Frankovič, A.; Ducman, V. Mechanical, microstructural and mineralogical evaluation of alkali-activated waste glass and stone wool. Ceram. Int. 2021, 47, 15102–15113. [Google Scholar] [CrossRef]
- Bagheri, A.; Moukannaa, S. A new approach to the reuse of waste glass in the production of alkali-activated materials. Clean. Eng. Technol. 2021, 4, 100212. [Google Scholar] [CrossRef]
- Rivera, J.; Cuarán-Cuarán, Z.; Vanegas-Bonilla, N.; Mejia, R. Novel use of waste glass powder: Production of geopolymeric tiles. Adv. Powder Technol. 2018, 29, 3448–3454. [Google Scholar] [CrossRef]
- Henao Rios, L.M.; Hoyos Triviño, A.F.; Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M. Effect of the use of waste glass (as precursor, and alkali activator) in the manufacture of geopolymer rendering mortars and architectural tiles. Constr. Build. Mater. 2023, 363, 129760. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, A.; Bao, X.; Ni, T.; Ling, J. A review on geopolymer in potential coating application: Materials, preparation and basic properties. J. Build. Eng. 2020, 32, 101734. [Google Scholar] [CrossRef]
- Christophliemk, M.P.; Pikkarainen, A.T.; Heponiemi, A.; Tuomikoski, S.; Runtti, H.; Hu, T.; Kantola, A.M.; Lassi, U. Preparation and characterization of porous and stable sodium- and potassium-based alkali activated material (AAM). Appl. Clay Sci. 2022, 230, 106697. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Dalvand, A.; Mastali, M.; Luukkonen, T.; Illikainen, M. Effects of waste ground glass and lime on the crystallinity and strength of geopolymers. Mag. Concr. Res. 2018, 71, 1218–1231. [Google Scholar] [CrossRef]
- Nasir, M.; Mahmood, A.H.; Bahraq, A.A. History, recent progress, and future challenges of alkali-activated binders—An overview. Constr. Build. Mater. 2024, 426, 136141. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhang, S. A review of glass ceramic foams prepared from solid wastes: Processing, heavy-metal solidification and volatilization, applications. Sci. Total Environ. 2021, 781, 146727. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. How does aluminium foaming agent impact the geopolymer formation mechanism? Cem. Concr. Compos. 2017, 80, 277–286. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass waste for development of Geopolymer composites—Mechanical properties and rheological characteristics: A review. Constr. Build. Mater. 2019, 220, 547–564. [Google Scholar] [CrossRef]
- Rincón, A.; Desideri, D.; Bernardo, E. Functional glass-ceramic foams from ‘inorganic gel casting’ and sintering of glass/slag mixtures. J. Clean. Prod. 2018, 187, 250–256. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Kashani, A. Sustainable one-part geopolymer foams with glass fines versus sand as aggregates. Constr. Build. Mater. 2018, 171, 223–231. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Menchaca-Ballinas, L.E.; Escalante-Garcia, J.I. Low CO2 emission cements of waste glass activated by CaO and NaOH. J. Clean. Prod. 2019, 239, 117992. [Google Scholar] [CrossRef]
- Mugahed Amran, Y.H.; Alyousef, R.; Alabduljabbar, H.; Khudhair, M.H.R.; Hejazi, F.; Alaskar, A.; Alrshoudi, F.; Siddika, A. Performance properties of structural fibred-foamed concrete. Results Eng. 2020, 5, 100092. [Google Scholar] [CrossRef]
- Si, R.; Guo, S.; Dai, Q.; Wang, J. Atomic-structure, microstructure and mechanical properties of glass powder modified metakaolin-based geopolymer. Constr. Build. Mater. 2020, 254, 119303. [Google Scholar] [CrossRef]
- Quercia, G.; Hüsken, G.; Brouwers, H.J.H. Water demand of amorphous nano silica and its impact on the workability of cement paste. Cem. Concr. Res. 2012, 42, 344–357. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, R.; Ma, Y.; Zhang, M.; Bai, Y.; Huang, B. Influence of waste glass powder on the physico-mechanical properties and microstructures of fly ash-based geopolymer paste after exposure to high temperatures. Constr. Build. Mater. 2020, 262, 120579. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Sahmaran, M. Extensive evaluation on the effect of glass powder on the rheology, strength, and microstructure of metakaolin-based geopolymer binders. Constr. Build. Mater. 2021, 268, 121168. [Google Scholar] [CrossRef]
- Shoaei, P.; Ameri, F.; Reza Musaeei, H.; Ghasemi, T.; Cheah, C.B. Glass powder as a partial precursor in Portland cement and alkali-activated slag mortar: A comprehensive comparative study. Constr. Build. Mater. 2020, 251, 118991. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag. 2016, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Li, H.; Zhu, H.; Liu, T.; Chen, Q.; Guo, H. Reuse of waste glass powder in alkali-activated metakaolin/fly ash pastes: Physical properties, reaction kinetics and microstructure. Resour. Conserv. Recycl. 2021, 173, 105721. [Google Scholar] [CrossRef]
- Huseien, G.F.; Hamzah, H.K.; Mohd Sam, A.R.; Khalid, N.H.A.; Shah, K.W.; Deogrescu, D.P.; Mirza, J. Alkali-activated mortars blended with glass bottle waste nano powder: Environmental benefit and sustainability. J. Clean. Prod. 2020, 243, 118636. [Google Scholar] [CrossRef]
- Liu, G.; Florea, M.V.A.; Brouwers, H.J.H. Waste glass as binder in alkali activated slag–fly ash mortars. Mater. Struct. 2019, 52, 101. [Google Scholar] [CrossRef]
- Si, R.; Dai, Q.; Guo, S.; Wang, J. Mechanical property, nanopore structure and drying shrinkage of metakaolin-based geopolymer with waste glass powder. J. Clean. Prod. 2020, 242, 118502. [Google Scholar] [CrossRef]
- Torres, J.; Palacios, M.; Hellouin, M.; Puertas, F. Alkaline chemical activation of urban glass wastes to produce cementitious Materials. In Proceedings of the 1st Spanish National Conference on Advances in Materials Recycling and Eco—Energy, Madrid, Spain, 12–13 November 2009. [Google Scholar]
- Kravchenko, E.; Lazorenko, G.; Jiang, X.; Leng, Z. Alkali-activated materials made of construction and demolition waste as precursors: A review. Sustain. Mater. Technol. 2024, 39, e00829. [Google Scholar] [CrossRef]
- Tho-In, T.; Sata, V.; Boonserm, K.; Chindaprasirt, P. Compressive strength and microstructure analysis of geopolymer paste using waste glass powder and fly ash. J. Clean. Prod. 2018, 172, 2892–2898. [Google Scholar] [CrossRef]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on concrete containing ground waste glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Dyer, T.; Dhir, R. Chemical reactions of glass cullet used as cement component. Am. Soc. Civ. Eng. 2001, 31, 412–417. [Google Scholar] [CrossRef]
- Nassar, R.-U.-D.; Soroushian, P. Strength and durability of recycled aggregate concrete containing milled glass as partial replacement for cement. Constr. Build. Mater. 2012, 29, 368–377. [Google Scholar] [CrossRef]
- Nassar, R.-U.-D.; Soroushian, P. Green and Durable Mortar Produced with Milled Waste Glass. Mag. Concr. Res. 2012, 64, 605–615. [Google Scholar] [CrossRef]
- Pourabbas Bilondi, M.; Toufigh, M.M.; Toufigh, V. Experimental investigation of using a recycled glass powder-based geopolymer to improve the mechanical behavior of clay soils. Constr. Build. Mater. 2018, 170, 302–331. [Google Scholar] [CrossRef]
- Hoy, M.; Rachan, R.; Horpibulsuk, S.; Arulrajah, A.; Mirzababaei, M. Effect of wetting–drying cycles on compressive strength and microstructure of recycled asphalt pavement—Fly ash geopolymer. Constr. Build. Mater. 2017, 144, 624–634. [Google Scholar] [CrossRef]
- Grubb, D.; Gallagher, P.; Wartman, J.; Liu, Y.; Carnivale, M. Laboratory Evaluation of Crushed Glass–Dredged Material Blends. J. Geotech. Geoenviron. Eng. 2006, 132, 562–576. [Google Scholar] [CrossRef]
- García-del-Toro, E.; Mas, M. Study of new formations of C-S-H in manufactured with glass powder as binder mortar. Ing. E Investig. 2018, 38, 24–32. [Google Scholar] [CrossRef]
- Xiao, R.; Polaczyk, P.; Zhang, M.; Jiang, X.; Zhang, Y.; Huang, B.; Hu, W. Evaluation of Glass Powder-Based Geopolymer Stabilized Road Bases Containing Recycled Waste Glass Aggregate. Transp. Res. Rec. J. Transp. Res. Board 2020, 2674, 22–32. [Google Scholar] [CrossRef]
- Ma, C.-K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Damage behavior of geopolymer composites exposed to elevated temperatures. Cem. Concr. Compos. 2008, 30, 986–991. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. (1980–2015) 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Tulyaganov, D.U.; Ferreira, J.M.F. Preparation and characterization of foams from sheet glass and fly ash using carbonates as foaming agents. Ceram. Int. 2009, 35, 229–235. [Google Scholar] [CrossRef]
- Mugoni, C.; Montorsi, M.; Siligardi, C.; Andreola, F.; Lancellotti, I.; Bernardo, E.; Barbieri, L. Design of glass foams with low environmental impact. Ceram. Int. 2015, 41 Pt A, 3400–3408. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Ji, R.; Liu, L.; Cheeseman, C.; Wang, X. Reuse of mineral wool waste and recycled glass in ceramic foams. Ceram. Int. 2019, 45, 15057–15064. [Google Scholar] [CrossRef]
- Ibrahim, J.E.F.M.; Tihtih, M.; Kurovics, E.; Gömze, L.A.; Kocserha, I. Innovative glass-ceramic foams prepared by alkali activation and reactive sintering of clay containing zeolite (zeolite-poor rock) and sawdust for thermal insulation. J. Build. Eng. 2022, 59, 105160. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Lye, C.Q.; Dhir, R.K. The role of glass waste in the production of ceramic-based products and other applications: A review. J. Clean. Prod. 2017, 167, 346–364. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Zhang, M.; Shen, X.; Petersen, R.R.; König, J.; Lee, P.D.; Yue, Y.; Cai, B. High-speed synchrotron X-ray imaging of glass foaming and thermal conductivity simulation. Acta Mater. 2020, 189, 85–92. [Google Scholar] [CrossRef]
- Singh, R.J.; Raut, A.; Murmu, A.L.; Jameel, M. Influence of Glass Powder Incorporated Foamed Geopolymer Blocks on Thermal and Energy Analysis of Building Envelope. J. Build. Eng. 2021, 43, 102520. [Google Scholar] [CrossRef]
- Flood, M.; Fennessy, L.; Lockrey, S.; Avendano, A.; Glover, J.; Kandare, E.; Bhat, T. Glass Fines: A review of cleaning and up-cycling possibilities. J. Clean. Prod. 2020, 267, 121875. [Google Scholar] [CrossRef]
- Righini, G.C. Glassy Microspheres for Energy Applications. Micromachines 2018, 9, 379. [Google Scholar] [CrossRef] [PubMed]















| Type of Waste Glass | Curing Time (h)/Temperature (°C) | Alkaline Activator | Activator Concentration (mol) | Major Findings | Ref |
|---|---|---|---|---|---|
| Glass bottles, window glass, fluorescent lamps | 24/70 | NaOH | 4, 6, 8, 10 | The ideal value for the activator (4–6 M), but as materials age, they lose strength due to phase shifts, breaking, and shrinkage. A C-S-H type gel was the primary reaction product. | [90] |
| Mixed-colour soda-lime glass | 20/85 | , KOH | - | A Si-high, Al-low gel was the primary reaction product, regardless of the nature of the activator or curing method. When alkaline-activator concentrations are high, less porous and more robust pastes with more compact microstructures are produced. | [33] |
| Glass wool, stone wool | 72/40 | 5 | The outcomes show that both precursors are appropriate for the alkali activation procedure. When sodium silicate or a solution of sodium silicate and NaOH was utilised, the compressive strength after three days of curing at 40 °C was superior in glass wool compared with stone wool. | [88] | |
| Window glass, hollow glass, windshield glass | 24/60 | KOH | 1, 2, 3, 5, 7, and 10 | The concentration of the activating solution and the time of the curing process at 60 °C both affect compressive strength. Regardless of the type of glass, 3 mol/L of activating solution (KOH) is the ideal concentration. | [20] |
| Pharmaceutical boro-aluminosilicate glass | -/40 | NaOH/KOH | 2.5 | When making alkaline solutions, industrial mud can be used in place of water. Products similar to facing bricks can be produced directly by cold consolidation or following the application of low-temperature (700 °C) firing, depending on the formulation. | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.J.; Elsayed, H.; Bernardo, E. Waste Glass Upcycling Supported by Alkali Activation: An Overview. Materials 2024, 17, 2169. https://doi.org/10.3390/ma17092169
Zafar MJ, Elsayed H, Bernardo E. Waste Glass Upcycling Supported by Alkali Activation: An Overview. Materials. 2024; 17(9):2169. https://doi.org/10.3390/ma17092169
Chicago/Turabian StyleZafar, Muhammad Jamshaid, Hamada Elsayed, and Enrico Bernardo. 2024. "Waste Glass Upcycling Supported by Alkali Activation: An Overview" Materials 17, no. 9: 2169. https://doi.org/10.3390/ma17092169






