Tunable Release of Calcium from Chitosan-Coated Bioglass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioglass Nanoparticle Synthesis
2.2. Chitosan Coating
2.3. Average Size and Zeta Potential
2.4. Transmission Electron Microscopy
2.5. Calcium Release Assays
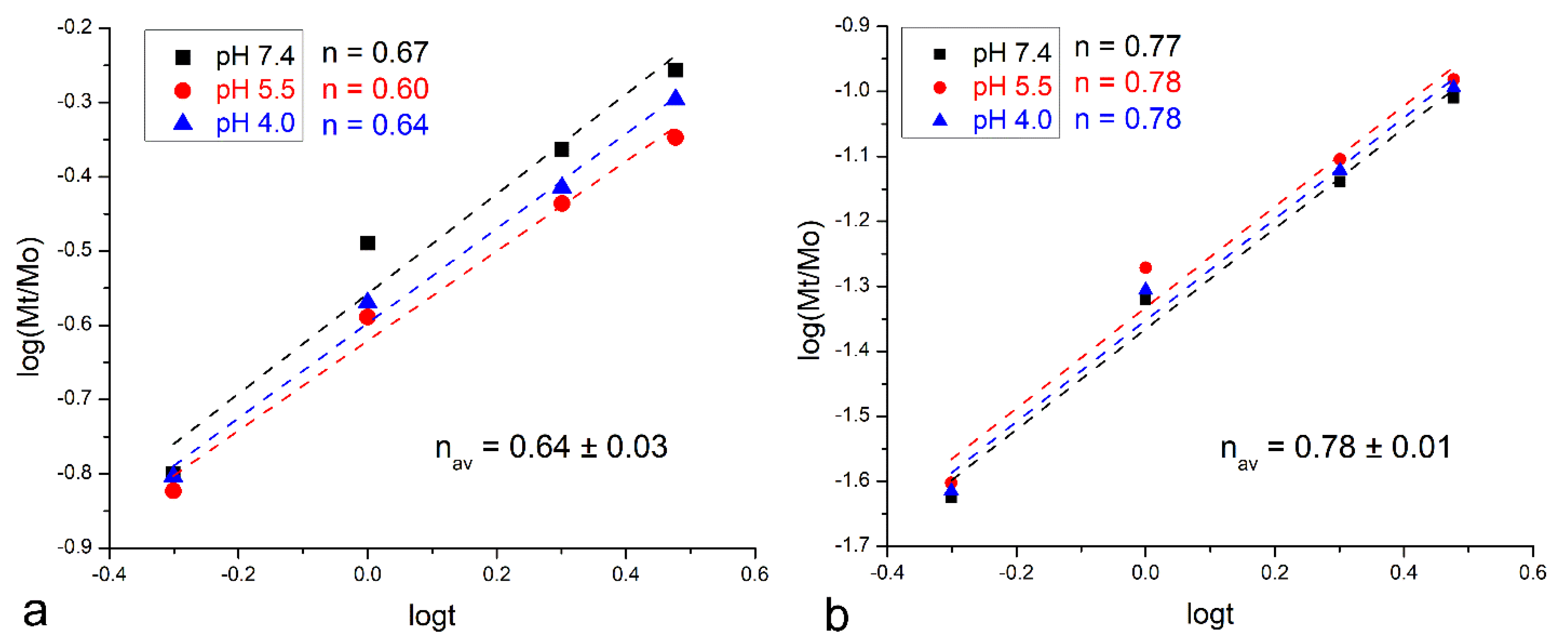
2.6. Kinetic Modeling
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murata, M.; Nezu, T.; Takebe, H.; Hirose, Y.; Okubo, N.; Saito, T.; Akazawa, T. Human dentin materials for minimally invasive bone regeneration: Animal studies and clinical cases. J. Oral Biosci. 2023, 65, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Mahendru, S.; Aggarwal, A.; Brajesh, V.; Aulakh, H.S.; Singh, S.; Jain, A.; Khazanchi, R.K. Feasibility of Customised Polymethyl Methacrylate Implants Fabricated Using 3D Printed Flexible Moulds for Correction of Facial Skeletal Deformities. J. Craniofac. Surg. 2021, 32, 1981–1985. [Google Scholar] [CrossRef] [PubMed]
- Raddall, G.; Mello, I.; Leung, B.M. Biomaterials and Scaffold Design Strategies for Regenerative Endodontic Therapy. Front. Bioeng. Biotechnol. 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.K.; Lim, G.S.; Singh, M.; Fial, A.V. Quantitative Assessment of root development after regenerative endodontic therapy: A systematic review and meta-analysis. J. Endod. 2020, 46, 1856–1866.e2. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Beckham, C.A.; Greenlee, T.K.; Crebo, A.R. Bone formation at a ceramic implant interface. Calcif. Tissue Int. 1971, 8, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Pigott, G.H.; Schoen, F.J.; Hench, L.L. Toxicology and biocompatibility of bioglasses. J. Biomed. Mater. Res. 1981, 15, 805–817. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Abuna, G.; Campos, P.; Hirashi, N.; Giannini, M.; Nikaido, T.; Tagami, J.; Sinhoreti, M.A.C.; Geraldeli, S. The ability of a nanobioglass-doped self-etching adhesive to re-mineralize and bond to artificially demineralized dentin. Dent. Mater. 2021, 37, 120–130. [Google Scholar] [CrossRef]
- Geissel, F.J.; Platania, V.; DeBerardinis, N.; Skjöldebrand, C.; Belibasakis, G.N.; Persson, C.; Hulsart-Billström, G.; Chatzinikolaidou, M.; Sotiriou, G.A. Nanostructured Ag-Bioglass Implant Coatings with Antibacterial and Osteogenic Activity. Adv. Mater. Interfaces 2023, 10, 2201980. [Google Scholar] [CrossRef]
- Yao, C.; Ahmed, M.H.; Li, X.; Nedeljkovic, I.; Vandooren, J.; Mercelis, B.; Zhang, F.; Van Landuyt, K.L.; Huang, C.; Van Meerbeek, B. Zinc–Calcium–Fluoride Bioglass-Based Innovative Multifunctional Dental Adhesive with Thick Adhesive Resin Film Thickness. ACS Appl. Mater. Interfaces 2020, 12, 30120–30135. [Google Scholar] [CrossRef]
- Skwira, A.; Szewczyk, A.; Sądej, R.; Prokopowicz, M. Bioglass obtained via one-pot synthesis as osseointegrative drug delivery system. Int. J. Pharm. 2023, 633, 122610. [Google Scholar] [CrossRef]
- Wetzel, R.; Bartzok, O.; Brauer, D.S. Influence of low amounts of zinc or magnesium substitution on ion release and apatite formation of Bioglass 45S5. J. Mater. Sci. Mater. Med. 2020, 31, 86. [Google Scholar] [CrossRef]
- Schmitz, S.; Widholz, B.; Essers, C.; Becker, M.; Tulyaganov, D.; Moghaddam, A.; de Juan, I.G.; Westhauser, F. Superior biocompatibility and comparable osteoinductive properties: Sodium-reduced fluoride-containing bioactive glass belonging to the CaO–MgO–SiO2 system as a promising alternative to 45S5 bioactive glass. Bioact. Mater. 2020, 5, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Ghosh, S. Carriers for the Tunable Release of Therapeutics: Etymological Classification and Examples. Expert Opin. Drug Deliv. 2016, 13, 1729–1741. [Google Scholar] [CrossRef]
- Schätzlein, E.; Kicker, C.; Söhling, N.; Ritz, U.; Neijhoft, J.; Henrich, D.; Frank, J.; Marzi, I.; Blaeser, A. 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering. Polymers 2022, 14, 2389. [Google Scholar] [CrossRef]
- Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Biodegradable Polymers. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2014; pp. 303–335. [Google Scholar]
- Kafetzopoulos, D.; Martinou, A.; Bouriotis, V. Bioconversion of chitin to chitosan: Purification and characterization of chitin deacetylase from Mucor rouxii. Proc. Natl. Acad. Sci. USA 1993, 90, 2564–2568. [Google Scholar] [CrossRef]
- Aiba, S.-I. Preparation of N-acetylchitooligosaccharides by hydrolysis of chitosan with chitinase followed by N-acetylation. Carbohydr. Res. 1994, 265, 323–328. [Google Scholar] [CrossRef]
- Tokuyasu, K.; Mitsutomi, M.; Yamaguchi, I.; Hayashi, K.; Mori, Y. Recognition of chitooligosaccharides and their N-acetyl groups by putative subsites of chitin deacetylase from a deuteromycete, Colletotrichum lindemuthianum. Biochemistry 2000, 39, 8837–8843. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Popat, A.; Liu, J.; Lu, G.Q.; Qiao, S.Z. A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 11173–11178. [Google Scholar] [CrossRef]
- Marudova, M.; Exner, G.; Pilicheva, B.; Marinova, A.; Viraneva, A.; Bodurov, I.; Sotirov, S.; Vlaeva, I.; Uzunova, Y. Effect of assembly pH and ionic strength of chitosan/casein multilayers on benzydamine hydrochloride release. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 90–98. [Google Scholar] [CrossRef]
- Zeng, X.; Ruckenstein, E. Control of Pore Sizes in Macroporous Chitosan and Chitin Membranes. Ind. Eng. Chem. Res. 1996, 35, 4169–4175. [Google Scholar] [CrossRef]
- Uskoković, V.; Desai, T.A. In vitro Analysis of Nanoparticulate Hydroxyapatite/Chitosan Composites as Potential Drug Delivery Platforms for the Sustained Release of Antibiotics in the Treatment of Osteomyelitis. J. Pharm. Sci. 2014, 103, 567–579. [Google Scholar] [CrossRef]
- Lin, A.; Chen, J.; Liu, Y.; Deng, S.; Wu, Z.; Huang, Y.; Ping, Q. Preparation and evaluation of N-caproyl chitosan nanoparticles surface modified with glycyrrhizin for hepatocyte targeting. Drug Dev. Ind. Pharm. 2009, 35, 1348–1355. [Google Scholar] [CrossRef]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA Polymer Blends as Coatings for Hydroxyapatite Nanoparticles and Their Effect on Antimicrobial Properties, Osteoconductivity and Regeneration of Osseous Tissues. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Fluorescence microscopy to follow the targeting of liposomes and micelles to cells and their intracellular fate. Adv. Drug Deliv. Rev. 2005, 57, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Labbaf, S.; Yaghini, J.; Talebi, A. Polymeric nanocomposite multifunctional core-shell membrane for periodontal repair and regeneration applications. Mater. Today Chem. 2022, 26, 101097. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Yang, X.; Han, C.; Gao, C.; Gou, Z. Bioactive glasses-incorporated, core–shell-structured polypeptide/polysaccharide nanofibrous hydrogels. Carbohydr. Polym. 2013, 92, 612–620. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Stamboulis, A.; Boccaccini, A.; Hench, L. Novel biodegradable polymer/bioactive glass composites for tissue engineering applications. Adv. Eng. Mater. 2002, 4, 105–109. [Google Scholar] [CrossRef]
- Boccaccini, A.; Blaker, J.; Maquet, V.; Day, R.; Jérôme, R. Preparation and characterisation of poly(lactide-co-glycolide) (PLGA) and PLGA/Bioglass® composite tubular foam scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2005, 25, 23–31. [Google Scholar] [CrossRef]
- Yao, J.; Radin, S.; Leboy, P.S.; Ducheyne, P. The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 2005, 26, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.; Chow, L.; Brown, W. A Microanalytical Procedure for the Determination of Calcium, Phosphate and Fluoride in Enamel Biopsy Samples. Caries Res. 1983, 17, 23–31. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carbohydr. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef]
- Uskokovic, V.; Lee, K.; Lee, P.P.; Fischer, K.E.; Desai, T.A. Shape Effect in the Design of Nanowire-Coated Microparticles as Epithelial Drug Delivery Devices. ACS Nano 2012, 6, 7832–7841. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Lee, P.P.; Walsh, L.; Fischer, K.E.; Desai, T.A. Silicon Nanowire Coated Microparticles as Epithelial Drug Delivery Devices. The Effect of PEGylation on Particle-Epithelium Interactions. Biomaterials 2012, 33, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, N.L.; Penov-Gaši, K.M.; Wu, V.M.; Ajduković, J.J.; Kojić, V.V.; Vasiljević-Radović, D.; Kuzmanović, M.; Uskoković, V.; Uskoković, D.P. Selective Anticancer Activity of Lung-Targeting Hydroxyapatite/Chitosan-Poly(D,L)-Lactide-co-Glycolide Particles Loaded with an Androstane-Based Cancer Inhibitor. Colloids Surf. B Biointerfaces 2016, 148, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Abuna, G.; Ferreira, P.; Wu, V.M.; Gower, L.; Pires-de-Souza, F.C.P.; Murata, R.M.; Sinhoreti, M.A.C.; Geraldeli, S. Synthesis and Characterization of Nanoparticulate Niobium- and Zinc-Doped Bioglass-Ceramic/Chitosan Hybrids for Dental Applications. J. Sol-Gel Sci. Technol. 2021, 97, 245–258. [Google Scholar] [CrossRef]
- Miyauchi, H.; Iwaku, M.; Fusayama, T. Physiological recalcification of carious dentin. Bull. Tokyo Med. Dent. Univ. 1978, 25, 169–179. [Google Scholar] [PubMed]
- Uskoković, V. Factors defining the stability of poly(lactide-co-glycolide) spheres for the sustained release of a cysteine protease inhibitor. Int. J. Pharm. Sci. 2020, 583, 119316. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Sakai, K.; Okano, T.; Sakurai, Y. Pulsatile drug delivery systems using hydrogels. Adv. Drug Deliv. Rev. 1993, 11, 85–108. [Google Scholar] [CrossRef]
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Miller, J.S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N. Bioresponsive Mesoporous Silica Nanoparticles for Triggered Drug Release. J. Am. Chem. Soc. 2011, 133, 19582–19585. [Google Scholar] [CrossRef]
- Alissawi, N.; Zaporojtchenko, V.; Strunskus, T.; Hrkac, T.; Kocabas, I.; Erkartal, B.; Chakravadhanula, V.S.K.; Kienle, L.; Grundmeier, G.; Garbe-Schönberg, D.; et al. Tuning of the ion release properties of silver nanoparticles buried under a hydrophobic polymer barrier. J. Nanopart. Res. 2012, 14, 928. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef]
- Tığlı Aydın, R.S.; Pulat, M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 313961. [Google Scholar] [CrossRef]
- Nair, R.; Paul, P.; Maji, I.; Gupta, U.; Mahajan, S.; Aalhate, M.; Guru, S.K.; Singh, P.K. Exploring the current landscape of chitosan-based hybrid nanoplatforms as cancer theragnostic. Carbohydr. Polym. 2024, 326, 121644. [Google Scholar] [CrossRef] [PubMed]
- Kariminia, S.; Shamsipur, M.; Barati, A. Fluorescent folic acid-chitosan/carbon dot for pH-responsive drug delivery and bioimaging. Int. J. Biol. Macromol. 2024, 254, 127728. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Zou, S.; Wei, Z.; Tong, Z. Simple, Reversible Emulsion System Switched by pH on the Basis of Chitosan without Any Hydrophobic Modification. Langmuir 2012, 28, 11017–11024. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uskoković, V.; Abuna, G.; Hampton, J.R.; Geraldeli, S. Tunable Release of Calcium from Chitosan-Coated Bioglass. Pharmaceutics 2024, 16, 39. https://doi.org/10.3390/pharmaceutics16010039
Uskoković V, Abuna G, Hampton JR, Geraldeli S. Tunable Release of Calcium from Chitosan-Coated Bioglass. Pharmaceutics. 2024; 16(1):39. https://doi.org/10.3390/pharmaceutics16010039
Chicago/Turabian StyleUskoković, Vuk, Gabriel Abuna, Joseph Ryan Hampton, and Saulo Geraldeli. 2024. "Tunable Release of Calcium from Chitosan-Coated Bioglass" Pharmaceutics 16, no. 1: 39. https://doi.org/10.3390/pharmaceutics16010039





