Elemental Uptake by Different Calcite Crystal Faces: An In Situ Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystallization Experiments
2.2. Analytical Techniques
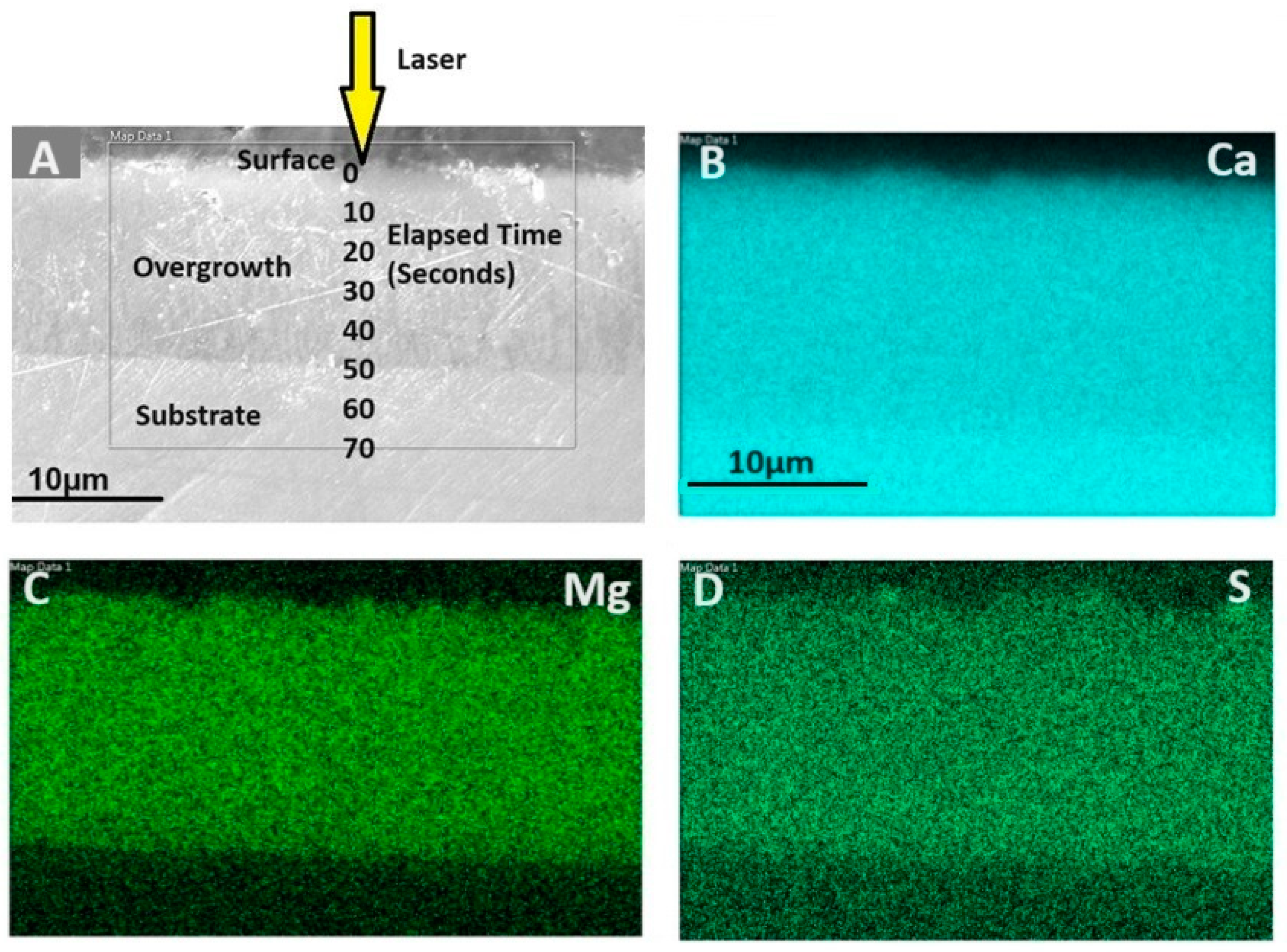
2.2.1. LA-ICP-MS (Depth Profiling)
2.2.2. SEM
3. Results and Discussions
3.1. Calcite Growth and Compositions
3.2. Evaluation of Growth Rate
3.3. Elemental Uptake by Calcite
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elderfield, H.; Ganssen, G. Past temperature and δ18O of surface ocean waters inferred from foraminiferal Mg/Ca ratios. Nature 2000, 405, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Riveiros, N.; Govin, A.; Waelbroeck, C.; Mackensen, A.; Michel, E.; Moreira, S.; Bouinot, T.; Caillon, N.; Orgun, A.; Brandon, M. Mg/Ca thermometry in planktic foraminifera: Improving paleotemperature estimations for G. bulloides and N. pachyderma left. Geochem. Geophys. Geosyst. 2016, 17, 1249–1264. [Google Scholar] [CrossRef]
- Pacho, L.; de Nooijer, L.; Reichart, G.-J. Element/Ca Ratios in Nodosariida (Foraminifera) and Their Potential Application for Paleoenvironmental Reconstructions. Biogeosciences 2023, 20, 4043–4056. [Google Scholar] [CrossRef]
- John, E.H.; Staudigel, P.T.; Buse, B.; Lear, C.H.; Pearson, P.N.; Slater, S.M. Revealing Their True Stripes: Mg/Ca Banding in the Paleogene Planktonic Foraminifera Genus Morozovella and Implications for Paleothermometry. Paleoceanogr. Paleoclimatology 2023, 38, e2023PA004652. [Google Scholar] [CrossRef]
- Salmon, K.H.; Anand, P.; Sexton, P.F.; Conte, M. Calcification and Growth Processes in Planktonic Foraminifera Complicate the Use of B/Ca and U/Ca as Carbonate Chemistry Proxies. Earth Planet. Sci. Lett. 2016, 449, 372–381. [Google Scholar] [CrossRef]
- Lombard, F.; Labeyrie, L.; Michel, E.; Spero, H.J.; Lea, D.W. Modelling the Temperature Dependent Growth Rates of Planktic Foraminifera. Mar. Micropaleontol. 2009, 70, 1–7. [Google Scholar] [CrossRef]
- Wanamaker Jr, A.D.; Kreutz, K.J.; Wilson, T.; Scourse, J.D.; Heinemeier, J.; Hughes, A.L.C.; Eiríksson, J.; Wild, B. Experimentally determined Mg/Ca and Sr/Ca ratios in juvenile bivalve calcite for Mytilus edulis: Implications for paleotemperature reconstructions. Geo-Mar. Lett. 2008, 28, 359–368. [Google Scholar] [CrossRef]
- Schleinkofer, N.; Raddatz, J.; Evans, D.; Gerdes, A.; Flögel, S.; Voigt, S.; Büscher, J.V.; Wisshak, M. Compositional variability of Mg/Ca, Sr/Ca, and Na/Ca in the deep-sea bivalve Acesta excavata (Fabricius, 1779). PLoS ONE 2021, 16, e0245605. [Google Scholar] [CrossRef] [PubMed]
- Strasser, C.A.; Mullineaux, L.S.; Thorrold, S.R. Temperature and salinity effects on elemental uptake in the shells of larval and juvenile softshell clams Mya arenaria. Mar. Ecol. Prog. Ser. 2008, 370, 155–169. [Google Scholar] [CrossRef]
- Dickson, J.A.D. Fossil Echinoderms As Monitor of the Mg/Ca Ratio of Phanerozoic Oceans. Science 2002, 298, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, S.; Mavromatis, V.; Buhl, D.; Dietzel, M.; Hoffmann, R.; Jöns, N.; Kell-Duivestein, I.; Immenhauser, A. Echinoid skeletal carbonate as archive of past seawater magnesium isotope signatures—Potential and limitations. Geochim. Cosmochim. Acta 2018, 235, 333–359. [Google Scholar] [CrossRef]
- Case, D.H.; Robinson, L.F.; Auro, M.E.; Gagnon, A.C. Environmental and biological controls on Mg and Li in deep-sea scleractinian corals. Earth Planet. Sci. Lett. 2010, 300, 215–225. [Google Scholar] [CrossRef]
- Saenger, C.; Gabitov, R.I.; Farmer, J.; Watkins, J.; Stone, R. Linear correlations in bamboo coral d13C and d18O sampled by SIMS and micromill: Evaluating paleoceanographic potential and biomineralization mechanisms using d11B and ∆47 variability. Chem. Geol. 2017, 454, 1–14. [Google Scholar] [CrossRef]
- Gagnon, A.C.; Adkins, J.F.; Fernandez, D.P.; Robinson, L.F. Sr/Ca and Mg/Ca vital effects correlated with skeletal architecture in a scleractinian deep-sea coral and the role of Rayleigh fractionation. Earth Planet. Sci. Lett. 2007, 261, 280–295. [Google Scholar] [CrossRef]
- Börner, N.; De Baere, B.; Yang, Q.; Jochum, K.P.; Frenzel, P.; Andreae, M.O.; Schwalb, A. Ostracod Shell Chemistry as Proxy for Paleoenvironmental Change. Quat. Int. 2013, 313–314, 17–37. [Google Scholar] [CrossRef]
- Rodríguez, M.; Not, C. Calibration of Mg/Ca and Sr/Ca in coastal marine ostracods as a proxy for temperature. Biogeosciences 2021, 18, 1987–2001. [Google Scholar] [CrossRef]
- Wansard, G.; De Deckker, P.; Julia, R. Variability in ostracod partition coefficients D Sr and D Mg: Implications for lacustrine palaeoenvironmental reconstructions. Chem. Geol. 1998, 146, 39–54. [Google Scholar] [CrossRef]
- Elmore, A.C.; Sosdian, S.; Rosenthal, Y.; Wright, J.D. A Global Evaluation of Temperature and Carbonate Ion Control on Mg/Ca Ratios of Ostracoda Genus Krithe. Geochem. Geophys. Geosyst. 2012, 13, Q09003. [Google Scholar] [CrossRef]
- Coggon, R.M.; Teagle, D.A.; Smith-Duque, C.E.; Alt, J.C.; Cooper, M.J. Reconstructing past seawater Mg/Ca and Sr/Ca from mid-ocean ridge flank calcium carbonate veins. Science 2010, 327, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Ligi, M.; Bonatti, E.; Cuffaro, M.; Brunelli, D. Post-Mesozoic Rapid Increase of Seawater Mg/Ca due to Enhanced Mantle-Seawater Interaction. Sci. Rep. 2013, 3, 2752. [Google Scholar] [CrossRef] [PubMed]
- Kısakürek, B.; James, R.H.; Harris, N.B.W. Li and δ7Li in Himalayan rivers: Proxies for silicate weathering? Earth Planet. Sci. Lett. 2005, 237, 387–401. [Google Scholar] [CrossRef]
- Hathorne, E.C.; James, R.H. Temporal record of lithium in seawater: A tracer for silicate weathering? Earth Planet. Sci. Lett. 2006, 246, 393–406. [Google Scholar] [CrossRef]
- Katz, A. The Interaction of Magnesium with Calcite during Crystal Growth at 25–90 °C and One Atmosphere. Geochim. Cosmochim. Acta 1973, 37, 1563–1586. [Google Scholar] [CrossRef]
- Mucci, A. Influence of temperature on the composition of magnesian calcite overgrowths precipitated from seawater. Geochim. Cosmochim. Acta 1987, 51, 1977–1984. [Google Scholar] [CrossRef]
- Spivack, A.J.; You, C.-F.; Smith, H.J. Foraminiferal boron isotope ratio as a proxy for surface ocean pH over the past 21 Myr. Nature 1993, 363, 149–151. [Google Scholar] [CrossRef]
- Sanyal, A.; Hemming, N.G.; Hanson, G.N.; Broecker, W.S. Evidence for a higher pH in the glacial ocean from boron isotopes in foraminifera. Nature 1995, 373, 234–236. [Google Scholar] [CrossRef]
- Sanyal, A.; Hemming, N.G.; Broecker, W.S.; Lea, D.W.; Spero, H.J.; Hanson, G.N. Oceanic pH control on the boron isotopic composition of foraminifera: Evidence from culture experiments. Paleoceanogr. Paleoclimatol. 1996, 11, 513–517. [Google Scholar] [CrossRef]
- Foster, G.L. Seawater pH, pCO2 and [CO32−] variations in the Caribbean Sea over the last 130 Kyr: A boron isotope and B/Ca study of planktic foraminifera. Earth Planet. Sci. Lett. 2008, 271, 254–266. [Google Scholar] [CrossRef]
- Mucci, A.; Morse, J.W. The incorporation of Mg2+ and Sr2+ into calcite overgrowths: Influences of growth rate and solution composition. Geochim. Cosmochim. Acta 1983, 47, 217–233. [Google Scholar] [CrossRef]
- Lorens, R.B. Sr, Cd, Mn, and Co distribution coefficients in calcite as a function of calcite precipitation rate. Geochim. Cosmochim. Acta 1981, 45, 553–561. [Google Scholar] [CrossRef]
- Tesoriero, A.J.; Pankow, J.F. Solid solution partition of Sr2+, Ba2+, and Cd2+ to calcite. Geochim. Cosmochim. Acta 1996, 60, 1053–1063. [Google Scholar] [CrossRef]
- Stoll, H.M.; Rosenthal, Y.; Falkowski, P. Climate proxies from Sr/Ca of coccolith calcite: Calibrations from continuous culture of Emiliania huxleyi. Geochim. Cosmochim. Acta 2002, 66, 927–936. [Google Scholar] [CrossRef]
- Tang, J.; Köhler, S.J.; Dietzel, M. Sr2+/Ca2+ and 44Ca/40Ca fractionation during inorganic calcite formation: I. Sr incorporation. Geochim. Cosmochim. Acta 2008, 72, 3718–3732. [Google Scholar] [CrossRef]
- Mavromatis, V.; Gautier, Q.; Bosc, O.; Schott, J. Kinetics of Mg partition and Mg stable isotope fractionation during its incorporation in calcite. Geochim. Cosmochim. Acta 2013, 114, 188–203. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Watson, E.B. Partitioning of strontium between calcite and fluid. Geochem. Geophys. 2006, 7, Q11004. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Rollion-Bard, C.; Tripati, A.; Sadekov, A. In situ study of boron partitioning between calcite and fluid at different crystal growth rates. Geochim. Cosmochim. Acta 2014, 137, 81–92. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Sadekov, A.; Leinweber, A. Crystal growth rate effect on Mg/Ca and Sr/Ca partitioning between calcite and fluid: An in-situ approach. Chem. Geol. 2014, 367, 70–82. [Google Scholar] [CrossRef]
- Paquette, J.; Reeder, R.J. Relationship between surface structure, growth mechanism, and trace element incorporation in calcite. Geochim. Cosmochim. Acta 1995, 59, 735–749. [Google Scholar] [CrossRef]
- Reeder, R.J. Interaction of divalent cobalt, zinc, cadmium, and barium with the calcite surface during layer growth. Geochim. Cosmochim. Acta 1996, 60, 1543–1552. [Google Scholar] [CrossRef]
- Hemming, N.G.; Hanson, G.N. Boron isotopic composition and concentration in modern marine carbonates. Geochim. Cosmochim. Acta 1992, 56, 537–543. [Google Scholar] [CrossRef]
- Shannon, R.D. Study on Revised Effective Ionic Radii in Halides and Chalcogenides. Acta Crystallogr. Sect. A Found. Crystallogr. 1976, A32, 751–767. [Google Scholar]
- Wasylenki, L.E.; Dove, P.M.; Wilson, D.S.; De Yoreo, J.J. Nanoscale effects of strontium on calcite growth: An in situ AFM study in the absence of vital effects. Geochim. Cosmochim. Acta 2005, 69, 3017–3027. [Google Scholar] [CrossRef]
- Wasylenki, L.E.; Dove, P.M.; Wilson, D.S.; De Yoreo, J.J. Effects of temperature and transport conditions on calcite growth in the presence of Mg2+: Implications for geothermometry. Geochim. Cosmochim. Acta 2005, 69, 4227–4236. [Google Scholar] [CrossRef]
- Hemming, N.G.; Reeder, R.J.; Hart, S.R. Growth-step selective incorporation of boron on the calcite surface. Geochim. Cosmochim. Acta 1998, 62, 2915–2922. [Google Scholar] [CrossRef]
- Payne, V.E.; Rickaby, R.E.M.; Benning, L.G.; Shaw, S. Calcite crystal growth orientation: Implications for trace metal uptake into coccoliths. Miner. Mag. 2008, 72, 269–272. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Borrelli, C.; Buettner, J.; Kirkland, B.; Skarke, A.; Trail, D.; Garner, B.; Testa, M.; Wahidi, M.; Hoff, C.; et al. Characterization of Carbonate Crust from Methane Seep on North Atlantic Margin. Minerals 2019, 9, 138. [Google Scholar] [CrossRef]
- Gabitov, R.; Sadekov, A.; Perez-Huerta, A.; Borrelli, C.; Rezaei, M. Elemental uptake by individual calcite crystals. In Proceedings of the 2022 Goldschmidt Conference, Honolulu, HI, USA, 11–15 July 2022. [Google Scholar]
- Zhong, S.; Mucci, A. Partitioning of rare earth elements (REEs) between calcite and seawater solutions at 25 °C and 1 atm, and high dissolved REE concentrations. Geochim. Cosmochim. Acta 1995, 59, 443–453. [Google Scholar] [CrossRef]
- Toyama, K.; Terakado, Y. Experimental study of rare earth element partitioning between calcite and sodium chloride solution at room temperature and pressure. Geochem. J. 2014, 48, 463–477. [Google Scholar] [CrossRef]
- Voigt, M.; Mavromatis, V.; Oelkers, E.H. The experimental determination of REE partition coefficients in the water-calcite system. Chem. Geol. 2017, 462, 30–43. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Sadekov, A.; Migdisov, A. REE incorporation into calcite individual crystals as one time spike addition. Minerals 2017, 7, 204. [Google Scholar] [CrossRef]
- Rezaei, M.; Gabitov, R.; Sadekov, A. Crystallographic influence on elemental uptake by calcite from artificial seawater. In Proceedings of the GSA Connects 2023, Pittsburgh, PA, USA, 15–18 October 2023; Geological Society of America: Boulder, CO, USA, 2023. Paper No. 254-11. [Google Scholar]
- Gabitov, R.I.; Sadekov, A.; Dyer, J.; Perez-Huerta, A.; Xu, H.; Migdisov, A. Sectoral and growth rate control on elemental uptake by individual calcite crystals. Chem. Geol. 2021, 585, 120589. [Google Scholar] [CrossRef]






| n | V Face 1, or 2 (µm/day) | s.e. | n | V Face 4 or 5 (µm/day) | s.e. | n | V Face 3 or 6 (µm/day) | s.e. | n | V, Cut (µm/day) | s.e. | n | V Total µm/day | s.e. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 0.097 | 0.019 | 5 | 0.121 | 0.015 | 5 | 0.111 | 0.004 | 3 | 0.119 | 0.010 | 17 | 0.112 | 0.027 |
| E/Ca (mmol/mol) | Face 1, 2 | s.e. | Face 4, 5 | s.e. | Face 3, 6 | s.e. | Cut | s.e. |
|---|---|---|---|---|---|---|---|---|
| Mg/Ca | 26.6470 | 0.1494 | 27.0829 | 0.5174 | 29.0462 * | 0.2989 | 26.9673 | 0.1955 |
| Sr/Ca | 0.9128 | 0.0122 | 0.8963 | 0.0056 | 0.8924 | 0.0068 | 0.8901 | 0.0162 |
| S/Ca | 44.7119 * | 0.3714 | 42.2531 | 0.4903 | 42.7114 | 0.6431 | 40.0656 * | 0.5528 |
| Ba/Ca | 0.0323 | 0.0004 | 0.0320 | 0.0004 | 0.0301 * | 0.0005 | 0.0297 * | 0.0011 |
| B/Ca | 0.0863 | 0.0031 | 0.0857 | 0.0027 | 0.0906 | 0.0015 | 0.0845 | 0.0017 |
| Li/Ca | 0.0103 | 0.0016 | 0.0103 | 0.0003 | 0.0132 * | 0.0004 | 0.0127 | 0.0006 |
| Na/Ca | 7.6817 | 0.6613 | 9.4596 | 1.4902 | 6.1757 * | 0.2046 | 6.9840 | 0.8160 |
| K/Ca | 0.9384 | 0.0548 | 0.9777 | 0.0626 | 0.9826 | 0.0261 | 0.8907 | 0.0403 |
| Experiment | Li/Ca | B/Ca | Mg/Ca | Sr/Ca | S/Ca | Ba/Ca | Na/Ca | K/Ca |
|---|---|---|---|---|---|---|---|---|
| F 1, or 2 | ×1.83 | ×1.17 | ×1.02 | ×1.06 | ×1.04 | ×1.05 | ×1.49 | ×1.32 |
| F 4, or 5 | ×1.16 | ×1.18 | ×1.11 | ×1.03 | ×1.07 | ×1.06 | ×2.18 | ×1.37 |
| F 3, or 6 | ×1.20 | ×1.09 | ×1.06 | ×1.04 | ×1.08 | ×1.08 | ×1.21 | ×1.15 |
| Cut | ×1.17 | ×1.07 | ×1.03 | ×1.06 | ×1.05 | ×1.13 | ×1.41 | x1.17 |
| Gabitov et al., 2021 [53] | ×10 | ×10 | ×3 | ×2 | - | - | - | - |
| Reeder, 1996 [39] | - | - | - | ×2 | - | ×2 | - | - |
| Hemming et al., 1998 [44] | - | ×9 | - | - | - | - | - | - |
| Paquette and Reeder, 1995 [38] | - | - | x7 | ×2 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaei, M.; Gabitov, R.; Sadekov, A.; Perez-Huerta, A.; Borrelli, C.; Stiles, A. Elemental Uptake by Different Calcite Crystal Faces: An In Situ Study. Crystals 2024, 14, 442. https://doi.org/10.3390/cryst14050442
Rezaei M, Gabitov R, Sadekov A, Perez-Huerta A, Borrelli C, Stiles A. Elemental Uptake by Different Calcite Crystal Faces: An In Situ Study. Crystals. 2024; 14(5):442. https://doi.org/10.3390/cryst14050442
Chicago/Turabian StyleRezaei, Mustafa, Rinat Gabitov, Aleksey Sadekov, Alberto Perez-Huerta, Chiara Borrelli, and Andrea Stiles. 2024. "Elemental Uptake by Different Calcite Crystal Faces: An In Situ Study" Crystals 14, no. 5: 442. https://doi.org/10.3390/cryst14050442






