Structural, Dielectric, Electrical, and Magnetic Characteristics of Bi0.8Ba0.1Er0.1Fe0.96Cr0.02Mn0.02O3 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Properties
3.1.1. Powder X-ray Diffraction (XRD)
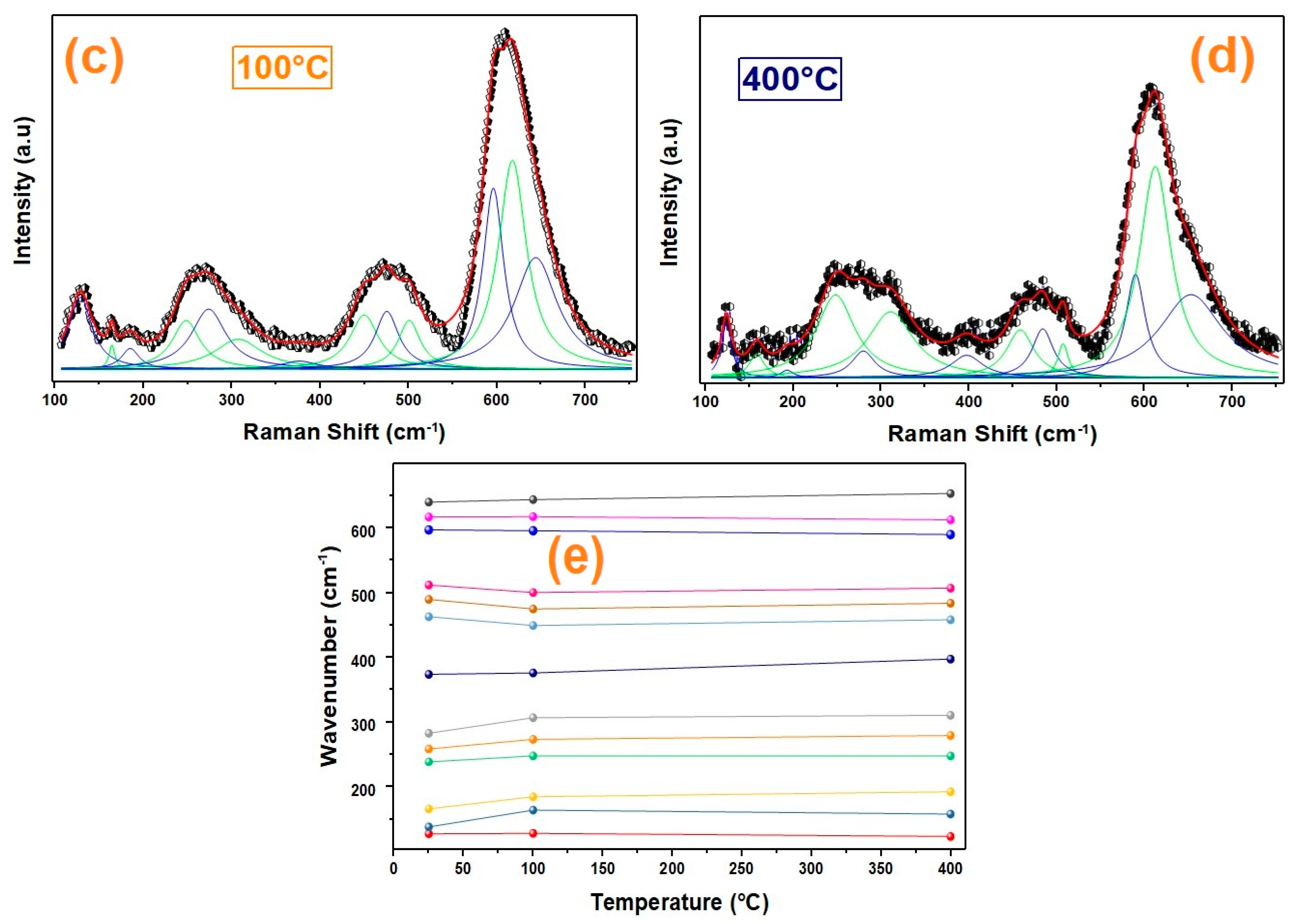
3.1.2. Raman Spectroscopy
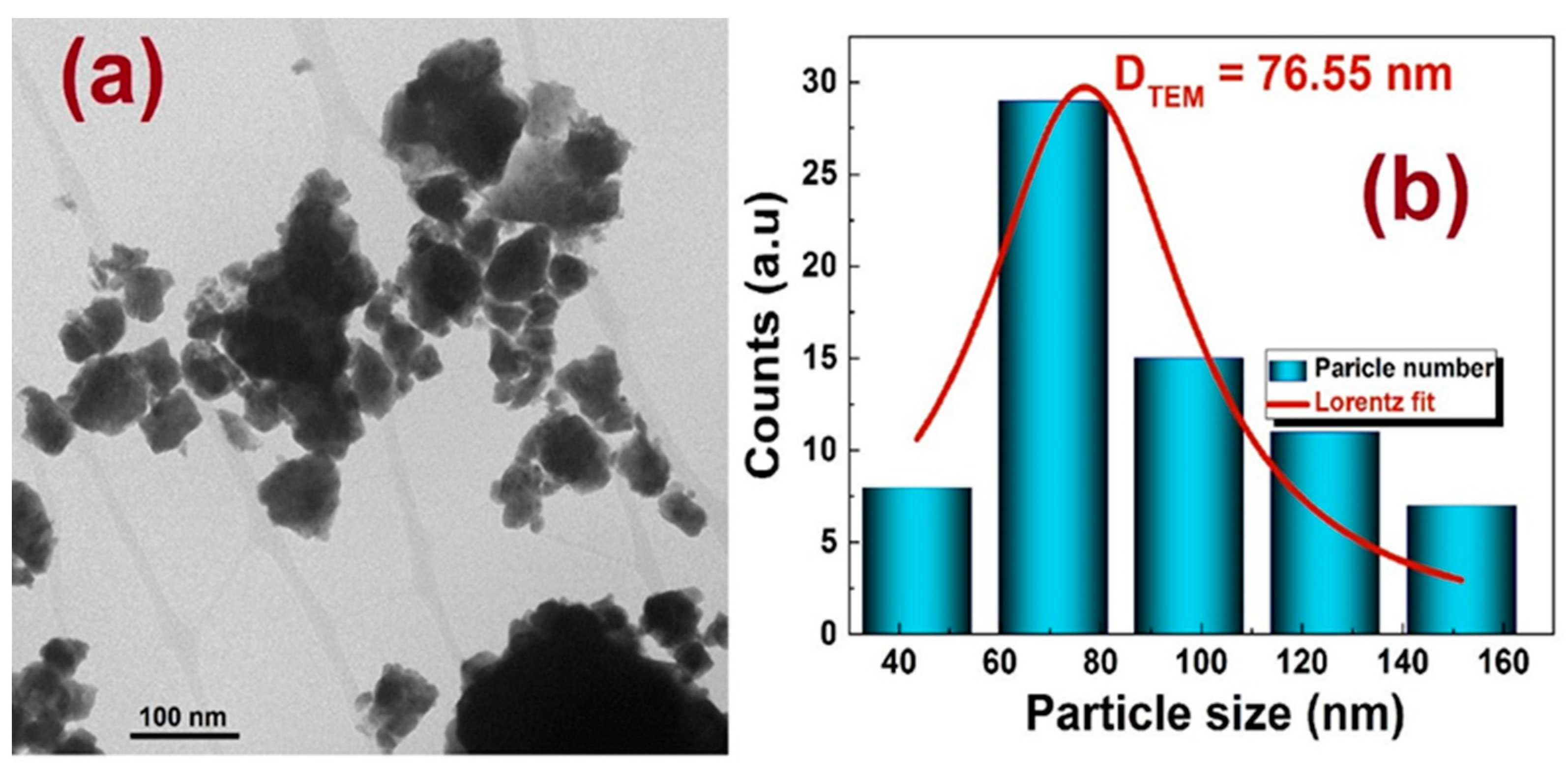
3.1.3. Transmission Electron Microscopy (TEM)
3.2. Magnetic Properties
3.3. Dielectric Study
3.3.1. Dielectric Constant and Dielectric Loss Tangent tg(δ)
3.3.2. Imaginary Part of Modulus
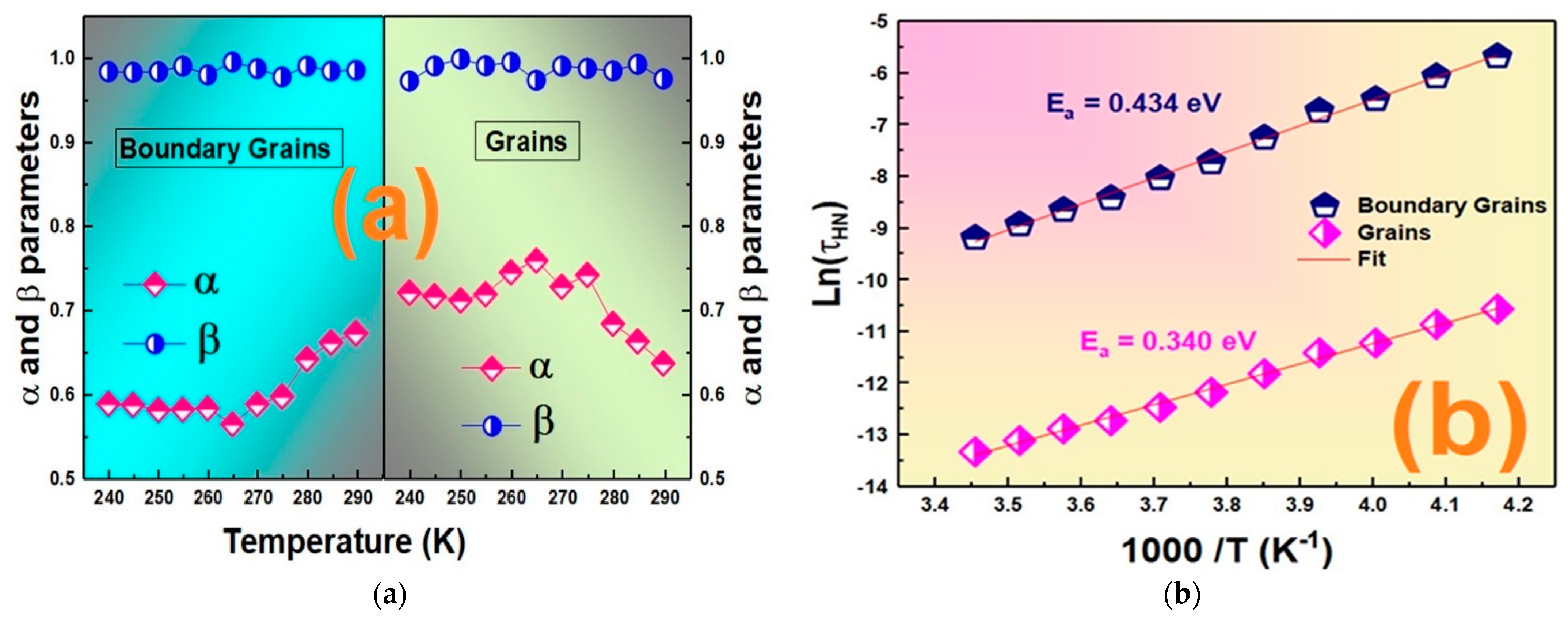
3.4. Electrical Investigation
3.4.1. DC Conductivity
3.4.2. AC Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheong, S.W.; Mostovoy, M. Multiferroics: A magnetic twist for ferroelectricity. Nat. Mater. 2007, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Prellier, W.; Singh, M.; Murugavel, P. The single-phase multiferroic oxides: From bulk to thin film. J. Phys. Condens. Matter. 2005, 17, 803. [Google Scholar] [CrossRef]
- Kumar, N.; Shukla, A.; Kumar, N.; Choudhary, R.N.P. Structural, electrical and magnetic properties of eco-friendly complex multiferroic material: Bi(Co0.35Ti0.35Fe0.30)O3. Ceram. Int. 2019, 45, 822–831. [Google Scholar] [CrossRef]
- Kumar, N.; Shukla, A.; Behera, C.; Choudhary, R.N.P. Structural, electrical and magnetic properties of Bi(Ni0.45Ti0.45Fe0.1)O3. J. Alloys Compd. 2016, 688, 858–869. [Google Scholar] [CrossRef]
- Arora, M.; Sati, P.C.; Chauhan, S.; Chhoker, S.; Panwar, A.K.; Kumar, M.J. Structural, Optical and Multiferroic Properties of BiFeO3 Nanoparticles Synthesized by Soft Chemical Route. Supercond. Nov. Magn. 2013, 26, 443. [Google Scholar] [CrossRef]
- Dutta, D.P.; Mandal, B.P.; Naik, R.; Lawes, G.; Tyagi, A.K. Magnetic, Ferroelectric, and Magnetocapacitive Properties of Sonochemically Synthesized Sc-Doped BiFeO3 Nanoparticles. J. Phys. Chem. C 2013, 117, 2382. [Google Scholar] [CrossRef]
- Fiebig, M. Revival of the magnetoelectric effect. J. Phys. D: Appl. Phys. 2005, 38, 123. [Google Scholar] [CrossRef]
- Shaheen, N.; Chen, Z.; Nong, Y.; Su, T.; Yousaf, M.; Lu, Y.; Li, L. Enhancing ORR Catalytic Activity and Electrochemical Investigation of La1−2xBaxBixFeO3 Cathode for Low-Temperature Solid Oxide Fuel Cell. Crystals 2023, 13, 822. [Google Scholar] [CrossRef]
- Sati, P.C.; Kumar, M.; Chhoker, S. Phase Evolution, Magnetic, Optical, and Dielectric Properties of Zr-Substituted Bi0.9Gd0.1FeO3 Multiferroics. J. Am. Ceram. Soc. 2015, 98, 1884–1890. [Google Scholar] [CrossRef]
- Ederer, C.; Spaldin, N.A. Influence of strain and oxygen vacancies on the magnetoelectric properties of multiferroic bismuth ferrite. Phys. Rev. B 2005, 71, 224103. [Google Scholar] [CrossRef]
- Das, S.R.; Choudhary, R.N.P.; Bhattacharya, P.; Katiyar, R.S. Structural and multiferroic properties of La-modified BiFeO3 ceramics. J. Appl. Phys. 2007, 101, 034104-7. [Google Scholar] [CrossRef]
- Sun, Q.; Fang, Y.; Wang, S.; Ma, W.; Zhang, Q.; Wang, J.; Wang, Y.; Lang, J.; Zhang, J.; Liu, Y.; et al. Mn dopant-enabled BiFeO3 with enhanced magnetic and photoelectric properties. Mater. Sci. Eng. B 2024, 299, 116921. [Google Scholar] [CrossRef]
- Yu, B.; Li, M.; Hu, Z.; Pei, L.; Guo, D.; Zhao, X.; Dong, S. Enhanced multiferroic properties of the high-valence Pr doped BiFeO3 thin film. Appl. Phys. Lett. 2008, 93, 182909-3. [Google Scholar] [CrossRef]
- Catalan, G.; Sardar, K.; Church, N.S.; Scott, J.F.; Harrison, R.J.; Redfern, S.A.T. Effect of chemical substitution on the Néel temperature of multiferroic Bi1−xCaxFeO3. Phys. Rev. B 2009, 79, 212415. [Google Scholar] [CrossRef]
- Jayakumar, O.D.; Achary, S.N.; Girija, K.G.; Tyagi, A.K.; Sudakar, C.; Lawes, G.; Naik, R.; Nisar, J.; Peng, X.; Ahuja, R. Theoretical and experimental evidence of enhanced ferromagnetism in Ba and Mn cosubstituted BiFeO3. J. Appl. Phys. Lett. 2010, 96, 032903. [Google Scholar] [CrossRef]
- Kumar, P.; Kar, M. Effect of structural transition on magnetic and optical properties of Ca and Ti co-substituted BiFeO3 ceramics. J. Alloys Compd. 2014, 584, 566–572. [Google Scholar] [CrossRef]
- Puhan, A.; Bhushan, B.; Kumar, V.; Panda, H.S.; Priyam, A.; Das, D.; Rout, D. Tailoring the structural, optical and magnetic properties of BiFeO3 multiferroic nanoparticles by Ba, Cr co-doping. Mater. Sci. Eng. B 2019, 241, 48–54. [Google Scholar] [CrossRef]
- Hoque, M.M.; Islam, M.T.; Islam, M.R.; Zubair, M.A. Effective bandgap tuning with non-trivial modulation in room temperature magnetic and electrical responses of low level Ba–Cr co-substituted BiFeO3 nanoparticles. Ceram. Int. 2022, 48, 19583–19596. [Google Scholar] [CrossRef]
- Rout, J.; Choudhary, R.N.P. Structural transformation and multiferroic properties of Ba–Mn co-doped BiFeO3. Phys. Lett. A 2016, 380, 288–292. [Google Scholar] [CrossRef]
- Wen, X.L.; Chen, Z.; Liu, E.H.; Lin, X.; Chen, C.L. Effect of Ba and Mn doping on microstructure and multiferroic properties of BiFeO3 ceramics. J. Alloys Comp. 2016, 678, 511–517. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, G.; Ren, X.; Li, J.; Xue, M.; Ren, H.; Xia, A.; Liu, W. Electric field dependence of ferroelectric stability in BiFeO3 thin films co-doped with Er and Mn. Ceram. Int. 2020, 46, 18690–18697. [Google Scholar] [CrossRef]
- Naeimi, A.S.; Dehghan, E.; Sanavi Khoshnoud, D.; Gholizadeh, A. Enhancment of ferromagnetism in Ba and Er co-doped BiFeO3 nanoparticles. J. Magn. Magn. Mater. 2015, 393, 502–507. [Google Scholar] [CrossRef]
- Palkar, V.R.; Kundaliya, D.C.; Malik, S.K. Effect of Mn substitution on magnetoelectric properties of bismuth ferrite system. J. Appl. Phys. 2003, 93, 4337–4339. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Das, K.; Sarkar, B.; Ghosh, S.; De, S.K.; Sinha, G.; Lahtinen, J. Enhanced magnetic and dielectric properties of Eu and Co co-doped BiFeO3 nanoparticles. Appl. Phys. Lett. 2012, 101, 042401. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, G.; Guo, M.; Chai, Z.; Lv, L.; Xue, M.; Ren, X.; Li, J.; Ren, H.; Xia, A. Multiferroic properties of La/Er/Mn/Co multi-doped BiFeO3 thin films. Ceram. Int. 2019, 45, 11765–11775. [Google Scholar] [CrossRef]
- Kebede, M.T.; Dillu, V.; Devi, S.; Chauhan, S. A comparative investigation of structural, magnetic and photocatalytic properties of pure, Ce-Ni and Cd-Ni co-doped BiFeO3 nanoparticles. Mater. Sci. Eng. B 2024, 301, 117188. [Google Scholar] [CrossRef]
- Dong, G.; Tan, G.; Luo, Y.; Liu, W.; Ren, H.; Xia, A. Influence of multi-element co-doping on structure and multiferroic properties of BiFeO3 thin films. Mater. Lett. 2014, 136, 314–317. [Google Scholar] [CrossRef]
- Liu, J.P.; Lv, Z.L.; Hou, Y.X.; Zhang, L.P.; Cao, J.P.; Wang, H.W.; Zhao, W.B.; Zhang, C.; Bai, Y.; Meng, K.K.; et al. Substantial reduction of leakage currents in La/Er/Zn/Ti multielement-doped BiFeO3 multiferroic thin films. Ceram. Int. 2022, 48, 17328–17334. [Google Scholar] [CrossRef]
- Chai, Z.; Tan, G.; Yue, Z.; Yang, W.; Guo, M.; Ren, H.; Xia, A.; Xue, M.; Liu, Y.; Lv, L. Ferroelectric properties of BiFeO3 thin films by Sr/Gd/Mn/Co multi-doping. J. Alloys Comp. 2018, 746, 677–687. [Google Scholar] [CrossRef]
- Benali, A.; Melo, B.M.G.; Prezas, P.R.; Bejar, M.; Dhahri, E.; Graça, M.P.F.; Valente, M.A.; Nogueira, B.A.; Costa, B.F.O. Structural, morphological, Raman and ac electrical properties of the multiferroic sol-gel made Bi0.8Er0.1Ba0.1Fe0.96Cr0.02Co0.02O3 material. J. Alloys Compd. 2019, 775, 304–315. [Google Scholar] [CrossRef]
- Bougoffa, A.; Benali, A.; Bejar, M.; Dhahri, E.; Graça, M.P.F.; Valente, M.A.; Bessais, L.; Costa, B.F.O. Mg-substitution effect on microstructure, dielectric relaxation and conduction phenomenon of Fe based perovskite nanomaterials. J. Alloys Compd. 2021, 856, 157425. [Google Scholar] [CrossRef]
- Benali, A.; Benali, E.M.; Gouadria, S.; Elhadi, M.; Dhahri, E.; Graça, M.P.F.; Valente, M.A.; Costa, B.F.O. Colossal dielectric constant with enhanced magnetization in the La3+ and Ca2+ co-doped BiFeO3 nanoparticles. J. Mater. Sci. Mater. Electron. 2022, 33, 16236–16250. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65. [Google Scholar] [CrossRef]
- Bougoffa, A.; Benali, E.M.; Benali, A.; Bejar, M.; Dhahri, E.; Graça, M.P.F.; Valente, M.A.; Otero-Irurueta, G.; Costa, B.F.O. Investigation of temperature and frequency dependence of the dielectric properties of multiferroic (L0.8Ca0.2)0.4Bi0.6FeO3 nanoparticles for energy storage application. RSC Adv. 2022, 12, 6907–6917. [Google Scholar] [CrossRef]
- Benali, E.M.; Benali, A.; Bejar, M.; Dhahri, E.; Graca, M.P.F.; Valente, M.A.; Costa, B.F.O. Structural, morphological, Raman, dielectric and electrical properties of La1−2xBaxBixFeO3 (0.00 ≤ x ≤ 0.20) compounds. RSC Adv. 2021, 11, 36148–36165. [Google Scholar] [CrossRef] [PubMed]
- Horchani, M.; Omri, A.; Seif Eddine, M.; Benali, A.; Tozri, A.; Dhahri, E.; Pavani, K.; Costa, B.F.O.; Graca, M.F.P. Ni–Cu–Co ferrite synthesized using the sol-gel method: Effects of the Cr3+ ion concentration on its structural, electrical, and dielectric properties. Appl. Phys. A 2022, 128, 947. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.; Zhang, M.F.; Chen, X.Y.; Liu, J.M.; Liu, Z.G. Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl. Phys. Lett. 2004, 84, 1731–1733. [Google Scholar] [CrossRef]
- Bernardo, M.S.; Jardiel, T.; Peiteado, M.; Mompean, F.J.; Garcia-Hernandez, M.; Garcia, M.A.; Villegas, M.; Caballero, A.C. Intrinsic compositional inhomogeneities in bulk Ti-doped BiFeO3: Microstructure development and multiferroic properties. Chem. Mater. 2013, 25, 1533. [Google Scholar] [CrossRef]
- Fisher, J.G.; Jang, S.H.; Park, M.S.; Sun, H.; Moon, S.H.; Lee, J.S.; Hussain, A. The Effect of niobium doping on the electrical properties of 0.4(Bi0.5 K0.5)TiO3-0.6BiFeO3 lead-free piezoelectric ceramics. Materials 2015, 8, 8183–8194. [Google Scholar] [CrossRef]
- Goncalves, N.S.; Carvalho, J.A.; Lima, Z.M.; Sasaki, J.M. Size–strain study of NiO nanoparticles by X-ray powder diffraction line broadening. Mater. Lett. 2012, 72, 36–38. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, W.; Khan, S.; Husain, S.; Ansari, A. Tailoring dielectric properties and multiferroic behavior of nanocrystalline BiFeO3 via Ni doping. J. Appl. Phys. 2018, 124, 164105. [Google Scholar] [CrossRef]
- Singh, M.K.; Jang, H.M.; Ryu, S.; Jo, M.H. Polarized Raman scattering of multiferroic BiFeO3 epitaxial films with rhombohedral R3c symmetry. J. Appl. Phys. Lett. 2006, 88, 42907. [Google Scholar] [CrossRef]
- Kothari, D.; Reddy, V.R.; Sathe, V.G.; Gupta, A.; Banerjee, A.; Awasthi, A.M. Raman scattering study of polycrystalline magnetoelectric BiFeO3. J. Magn. Magn. Mater. 2008, 320, 548–552. [Google Scholar] [CrossRef]
- Xue, X.; Tan, G.; Liu, W.; Ren, H. Mater. Nd doping effect on Bi1−xNdxFe0.97Co0.03O3 thin films: Microstructural, electrical, optical and enhanced multiferroic properties. Chem. Phys. 2014, 146, 183–191. [Google Scholar] [CrossRef]
- Kumar, M.; Sati, P.C.; Chhoker, S. Electron spin resonance study and improved magnetic and dielectric properties of Gd–Ti co-substituted BiFeO3 ceramics. J. Mater. Sci. 2014, 25, 5366–5374. [Google Scholar] [CrossRef]
- Manjusha, E.; Maneesh, C. Raman study of BiFeO3 nanostructures using different excitation wavelengths: Effects of crystallite size on vibrational modes. J. Phys. Chem. Solids 2023, 172, 111060. [Google Scholar] [CrossRef]
- Reetu, R.; Agarwal, A.; Sanghi, S.; Ashima, A.; Ahlawat, N.; Monica, M. Phase transformation, dielectric and magnetic properties of Nb doped Bi0.8Sr0.2FeO3 multiferroics. J. Appl. Phys. 2012, 111, 113917. [Google Scholar] [CrossRef]
- Arafat, S.S. Structural transition and magnetic properties of high Cr-doped BiFeO3 ceramic. Cerâmica 2020, 66, 114–118. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, K. Magnetic, magnetocapacitance and dielectric properties of Cr doped bismuth ferrite nanoceramics. Mater. Sci. Eng. B 2011, 176, 227–230. [Google Scholar] [CrossRef]
- Wang, B.; Tian, X.; Song, X.; Ma, L.; Yu, S.; Hao, C.; Chen, K.; Lie, Q. Smart electrorheological behavior of Cr-doped multiferroelectric FeBiO3 nanoparticles. Colloid Surf. A 2014, 461, 184. [Google Scholar] [CrossRef]
- Layek, S.; Verma, H.C. Magnetic and dielectric properties of Multiferroic BiFeO3 nanoparticles synthesized by a novel citrate combustion method. Adv. Mater. Lett. 2012, 3, 533–538. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, Y.; Zhang, W.; Guo, C.; Zhang, X.; Zhao, J.; Zhang, Y.; Zheng, H. Optical and magnetic properties of Sm-doped BiFeO3 nanoparticles around the morphotropic phase boundary region. AIP Adv. 2021, 11, 045223. [Google Scholar] [CrossRef]
- Vijayasundaram, S.V.; Suresh, G.; Mondal, R.A.; Kanagadurai, R. Substitution-driven enhanced magnetic and ferroelectric properties of BiFeO3 nanoparticles. J. Alloys Compd. 2016, 658, 726–731. [Google Scholar] [CrossRef]
- Mao, W.; Wang, X.; Chu, L.; Zhu, Y.; Wang, Q.; Zhang, J.; Yang, J.; Li, X.; Huang, W. Simultaneous enhancement of magnetic and ferroelectric properties in Dy and Cr co-doped BiFeO3 nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 6399–6405. [Google Scholar] [CrossRef] [PubMed]
- Benali, A.; Bejar, M.; Dhahri, E.; Hlil, E.; Graça, M.P.F.; Valente, M.A.; Costa, B.F.O. Assessment of the critical behavior in the multiferroic Bi0.8Ba0.1Er0.1Fe0.96Cr0.02Co0.02O3 material, multi-substitution effect on magnetic and Mössbauer properties. J. Magn. Magn. Mater. 2021, 524, 167640. [Google Scholar] [CrossRef]
- Awasthi, R.R.; Das, B. Structural transition and tunable optical, morphological and magnetic properties of Mn-doped BiFeO3 films. Optic 2019, 194, 162973. [Google Scholar] [CrossRef]
- Dong, G.H.; Tan, G.Q.; Luo, Y.Y.; Liu, W.L.; Xia, A.; Ren, H.J. Charge defects and highly enhanced multiferroic properties in Mn and Cu co-doped BiFeO3 thin films. Appl. Surf. Sci. 2014, 305, 55–61. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Wang, C.; An, X.; Wang, Y.; Xu, W.; Gu, L. Magnetic and structure property correlations in Mn-doped BiFeO3 system. J. Mater Sci. Mater. Electron. 2020, 31, 13150–13157. [Google Scholar] [CrossRef]
- Shah, L.R.; Zhu, H.; Wang, W.G.; Ali, B.; Zhu, T.; Fan, X.; Song, Y.Q.; Wen, Q.Y.; Zhang, H.W.; Shah, S.I.; et al. Effect of Zn interstitials on the magnetic and transport properties of bulk Co-doped ZnO. J. Phys. D Appl. Phys. 2010, 43, 035002. [Google Scholar] [CrossRef]
- Kumar, P.; Shankhwar, N.; Srinivasan, A.; Kar, M. Oxygen octahedra distortion induced structural and magnetic phase transitions in Bi1−xCaxFe1−xMnxO3 ceramics. J. Appl. Phys. 2015, 117, 194103. [Google Scholar] [CrossRef]
- Bunget, I.; Popescu, M. Physics of Solid Dielectrics; Elsevier: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA; Tokyo, Japan, 1984. [Google Scholar]
- Hamam, K.J.; Salman, F. Dielectric constant and electrical study of solid-state electrolyte lithium phosphate glasses. Appl. Phys. A 2019, 125, 621. [Google Scholar] [CrossRef]
- Sinha, A.K.; Bhushan, B.; Jagannath; Sharma, R.K.; Sen, S.; Mandal, B.P.; Meena, S.S.; Bhatt, P.; Prajapat, C.L.; Priyam, A.; et al. Enhanced dielectric, magnetic and optical properties of Cr-doped BiFeO3 multiferroic nanoparticles synthesized by sol-gel route. Results Phys. 2019, 13, 102299. [Google Scholar] [CrossRef]
- Ke, S.; Fan, H.; Huang, H. Dielectric relaxation in A2FeNbO6 (A = Ba, Sr, and Ca) perovskite ceramics. J. Electroceram. 2007, 22, 252–256. [Google Scholar] [CrossRef]
- Ke, S.; Huang, H.; Fan, H.; Chan, H.L.W.; Zhou, L.M. Colossal dielectric response in barium iron niobate ceramics obtained by different precursors. Ceram. Int. 2008, 34, 1059–1062. [Google Scholar] [CrossRef]
- Jumpatam, J.; Thongbai, P.; Yamwong, T.; Maensiri, S. Effects of Bi3+ doping on microstructure and dielectric properties of CaCu3Ti4O12/CaTiO3 composite ceramics. Ceram. Int. 2015, 41, S498–S503. [Google Scholar] [CrossRef]
- Pribosic, I.; Makovec, D.; Drofenic, M. Electrical properties of donor and acceptor doped BaBi4Ti4O15. Eur. Ceram. Soc. 2001, 21, 1327–1331. [Google Scholar] [CrossRef]
- Matin, M.A.; Hossain, M.N.; Ali, M.A.; Hakim, M.A.; Islam, M.F. Enhanced dielectric properties of prospective Bi0.85Gd0.15Fe1−xCrxO3 multiferroics. Results Phys. 2019, 12, 1653–1659. [Google Scholar] [CrossRef]
- Liu, X.; Fang, B.J.; Deng, J.; Deng, H.; Yan, H.; Yue, Q.W.; Chen, J.W.; Li, X.B.; Ding, J.N.; Zhao, X.Y.; et al. Phase transition behavior and defect chemistry of [001]-oriented 0.15Pb(In½Nb½)O3−0.57Pb(Mg1/3Nb2/3)O3−0.28PbTiO3-Mn single crystals. J. Appl. Phys. 2015, 117, 244102. [Google Scholar] [CrossRef]
- Kremer, F.; Schonhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Brazzano, L.C.; Sorichetti, P.A.; Santiago, G.D.; González, M.G. Broadband dielectric characterization of piezoelectric poly (vinylidene fluoride) thin films between 278 K and 308 K. Polym. Test. 2013, 32, 1186–1191. [Google Scholar] [CrossRef]
- Cole, K.; Cole, R. Dispersion and absorption in dielectrics. I: Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–352. [Google Scholar] [CrossRef]
- Davidson, D.; Cole, R. Dielectric relaxation in glycerol, propylene glycol, and n-propanol. J. Chem. Phys. 1951, 19, 1484–1490. [Google Scholar] [CrossRef]
- Moualhi, Y.; Nofal, M.M.; M’nassri, R.; Rahmouni, H.; Selmi, A.; Gassoumi, M.; Khirouni, K.; Cheikrouhou, A. Double Jonscher response and contribution of multiple mechanisms in electrical conductivity processes of Fe-PrCaMnO ceramic. Ceram. Int. 2020, 46, 1601. [Google Scholar] [CrossRef]
- Mott, N.F. The origin of some ideas on non-crystalline materials. J. Non-Cryst. Solids 1978, 28, 147. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in glasses containing transition metal ions. J. Non-Cryst. Solids 1968, 1, 1. [Google Scholar] [CrossRef]
- Austin, I.G.; Mott, N.F. Polarons in crystalline and non-crystalline materials. Adv. Phys. 1969, 18, 41. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in non-crystalline materials. Phil. Mag. 1969, 19, 835. [Google Scholar] [CrossRef]
- Mott, N.F. Polarons. Mater. Res. Bull. 1978, 13, 1389. [Google Scholar] [CrossRef]
- Greaves, G.N. Small polaron conduction in V2O5 P2O5 glasses. J. Non-Cryst. Solids 1973, 11, 427. [Google Scholar] [CrossRef]
- Jonscher, A.K. Universal Relaxation Law; Chelsea Dielectric Press: London, UK, 1996. [Google Scholar]









| Lattice Parameters | ||||
| a = b (Å) | 5.561 (3) | |||
| c (Å) | 13.769 (3) | |||
| V (Å3) | 368.818 (12) | |||
| Interatomic distances | ||||
| Fe/Cr/Mn–O (Å) | 2.2861/1.8025 | |||
| Bi/Er/Ba–O (Å) | 2.6444/2.1023 | |||
| Fe–O–Fe (°) | 149.431 | |||
| R-factors (%) | ||||
| Rwp | 4.873 | |||
| Rexp | 3.132 | |||
| GOF | 1.555 | |||
| χ2 | 2.420 | |||
| Atomic position parameters | ||||
| Atom | x | y | z | |
| Bi/Er/Ba | 0 | 0 | 0.2206 | |
| Fe/Cr/Mn | 0 | 0 | 0 | |
| O | 0.8812 | 0.6558 | 0.4533 | |
| Raman Mode [21] | 25 °C/(cm−1) | 100 °C/(cm−1) | 400 °C/(cm−1) |
|---|---|---|---|
| A1-1 | 127.90 | 128.57 | 123.67 |
| A1-2 | 138.22 | 164.27 | 158.15 |
| A1-3 | 166.28 | 184.96 | 192.74 |
| A1-4 | 238.88 | 248.22 | 248.15 |
| E | 258.74 | 273.73 | 279.74 |
| E | 283.14 | 307.18 | 310.92 |
| E | 374.10 | 376.39 | 397.65 |
| E | 463.25 | 449.73 | 458.66 |
| E | 490.03 | 475.36 | 484.28 |
| E | 512.37 | 500.71 | 507.51 |
| E | 597.08 | 595.86 | 589.96 |
| E | 617.02 | 617.50 | 612.65 |
| E | 640.04 | 643.85 | 653.32 |
| Observed Magnetization (emu/g) | AFM Contribution (×105 emu*Oe/g) | FM Contribution | ||
|---|---|---|---|---|
| HCI (kOe) | MFMS (emu/g) | MFMR (emu/g) | ||
| 8.051 | 0.325 | 4.208 | 4.801 | 1.947 |
| Site | δ (mm/s) | Δ (mm/s) | B (T) | Γ (mm/s) | % | Coordination |
|---|---|---|---|---|---|---|
| Doublet | 0.23 (1) | 0.550 (1) | - | 0.47 (1) | 5.3 | Fe3+ |
| Sext 1 | 0.39 (1) | 0.034 (1) | 50.7 (1) | 0.45 (1) | 42.3 | Fe3+ |
| Sext 2 | 0.38 (1) | 0.032 (1) | 49.3 (1) | 0.47 (1) | 47.2 | Fe3+ |
| Sext 3 | 0.25 (1) | 0.049 (1) | 41.5 (1) | 0.67 (1) | 5.2 | Fe3+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougoffa, A.; Benali, E.M.; Benali, A.; Tozri, A.; Dhahri, E.; Graça, M.P.; Valente, M.A.; Costa, B.F.O. Structural, Dielectric, Electrical, and Magnetic Characteristics of Bi0.8Ba0.1Er0.1Fe0.96Cr0.02Mn0.02O3 Nanoparticles. Crystals 2024, 14, 445. https://doi.org/10.3390/cryst14050445
Bougoffa A, Benali EM, Benali A, Tozri A, Dhahri E, Graça MP, Valente MA, Costa BFO. Structural, Dielectric, Electrical, and Magnetic Characteristics of Bi0.8Ba0.1Er0.1Fe0.96Cr0.02Mn0.02O3 Nanoparticles. Crystals. 2024; 14(5):445. https://doi.org/10.3390/cryst14050445
Chicago/Turabian StyleBougoffa, A., E. M. Benali, A. Benali, A. Tozri, E. Dhahri, M. P. Graça, M. A. Valente, and B. F. O. Costa. 2024. "Structural, Dielectric, Electrical, and Magnetic Characteristics of Bi0.8Ba0.1Er0.1Fe0.96Cr0.02Mn0.02O3 Nanoparticles" Crystals 14, no. 5: 445. https://doi.org/10.3390/cryst14050445





