Silver Nanoparticle–PEDOT:PSS Composites as Water-Processable Anodes: Correlation between the Synthetic Parameters and the Optical/Morphological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Silver Nanoparticles in Presence of PEDOT:PSS
2.3. Characterization Techniques
2.4. Layer Deposition and Prototype Fabrication
3. Results and Discussion
3.1. Characterization of Silver Nanoparticles Stabilized with PEDOT:PSS (PP@AgNP) Suspensions
3.2. Deposition of the PP@AgNP Suspensions
3.2.1. Effect of Reducing Agent/AgNO3 Ratio
3.2.2. Effect of the Formulation of PEDOT:PSS Suspension
3.2.3. Effect of the AgNO3 and PEDOT:PSS Concentration in the Reaction Medium
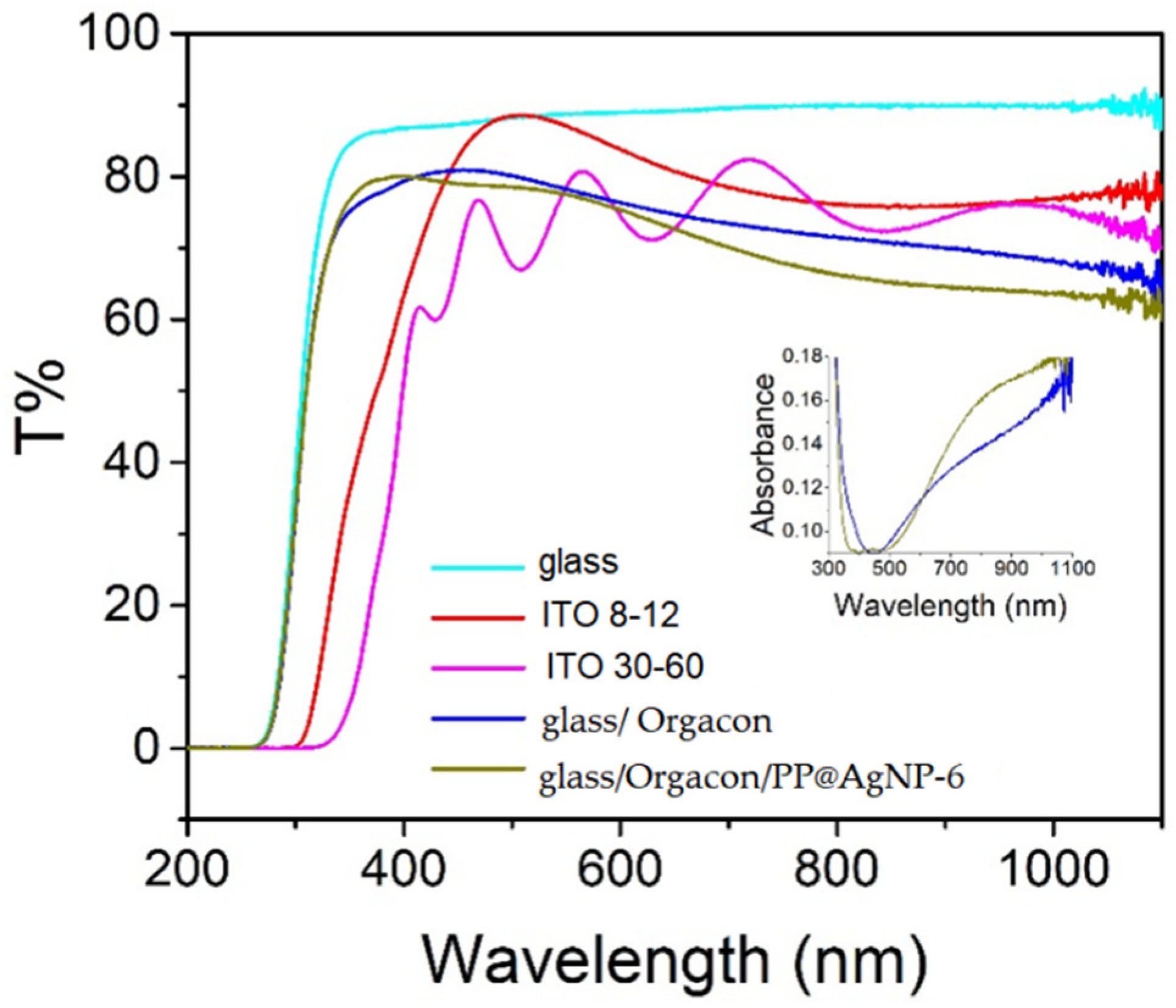
3.3. Solar Cells Prototypes: Preliminary Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Zhang, Y.; Zheng, Z. Metal-Based Flexible Transparent Electrodes: Challenges and Recent Advances. Adv. Electron. Mater. 2021, 7, 2001121. [Google Scholar] [CrossRef]
- Hu, L.; Song, J.; Yin, X.; Su, Z.; Li, Z. Research Progress on Polymer Solar Cells Based on PEDOT:PSS Electrodes. Polymers 2020, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Li, Q.; Li, G.; Tian, X.; Luo, Z.; Qiao, F.; Wu, X.; Zhang, J. Review of Printed Electrodes for Flexible Devices. Front. Mater. 2019, 5, 77. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.P. Progress in Understanding Structure and Transport Properties of PEDOT-Based Materials: A Critical Review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- EP0339340A2; Neue Polythiophene, Verfahren Zu Ihrer Herstellung und Ihre Verwendung. European Patent Office: Munich, Germany, 1988.
- Jonas, F.; Morrison, J.T. 3,4-Polyethylenedioxythiophene (PEDT): Conductive Coatings Technical Applications and Properties. Synth. Met. 1997, 85, 1397–1398. [Google Scholar] [CrossRef]
- Lang, U.; Muller, E.; Naujoks, N.; Dual, J. Microscopical Investigations of PEDOT:PSS Thin Films. Adv. Funct. Mater. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Dupont, S.R.; Novoa, F.; Voroshazi, E.; Dauskardt, R.H. Decohesion Kinetics of PEDOT:PSS Conducting Polymer Films. Adv. Funct. Mater. 2014, 24, 1325–1332. [Google Scholar] [CrossRef]
- Stocker, T.; Kohler, A.; Moos, R. Why Does the Electrical Conductivity in PEDOT:PSS Decrease with PSS Content? A Study Combining Thermoelectric Measurements with Impedance Spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 976–983. [Google Scholar] [CrossRef]
- Jeong, W.; Gwon, G.; Ha, J.H.; Kim, D.; Eom, K.J.; Park, J.H.; Kang, S.J.; Kwak, B.; Hong, J.-I.; Lee, S.; et al. Enhancing the Conductivity of PEDOT:PSS Films for Biomedical Applications via Hydrothermal Treatment. Biosens. Bioelectron. 2021, 171, 112717. [Google Scholar] [CrossRef]
- Ouyang, L.; Musumeci, C.; Jafari, M.J.; Ederth, T.; Inganäs, O. Imaging the Phase Separation between PEDOT and Polyelectrolytes during Processing of Highly Conductive PEDOT:PSS Films. ACS Appl. Mater. Interfaces 2015, 7, 19764–19773. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ballantyne, A.M.; Nelson, J.; Bradley, D.D.C. Effects of Thickness and Thermal Annealing of the PEDOT:PSS Layer on the Performance of Polymer Solar Cells. Org. Electron. 2009, 10, 205–209. [Google Scholar] [CrossRef]
- Fan, X.; Xu, B.; Liu, S.; Cui, C.; Wang, J.; Yan, F. Transfer-Printed PEDOT:PSS Electrodes Using Mild Acids for High Conductivity and Improved Stability with Application to Flexible Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 14029–14036. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kang, H.; Lee, J.H.; Kee, S.; Lee, S.H.; Lee, K. Highly Conductive All-Plastic Electrodes Fabricated Using a Novel Chemically Controlled Transfer-Printing Method. Adv. Mater. 2015, 27, 2317–2323. [Google Scholar] [CrossRef]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly Conductive PEDOT:PSS Nanofibrils Induced by Solution-Processed Crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-Processed Metallic Conducting Polymer Films as Transparent Electrode of Optoelectronic Devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef]
- Kim, Y.U.; Park, S.H.; Nhan, N.T.; Hoang, M.H.; Cho, M.J.; Choi, D.H. Optimal Design of PEDOT:PSS Polymer-Based Silver Nanowire Electrodes for Realization of Flexible Polymer Solar Cells. Macromol. Res. 2021, 29, 75–81. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, M.; Kim, S.S.; Paek, S.H.; Kim, Y.C. Silver Nanowire/PEDOT:PSS Hybrid Electrode for Flexible Organic Light-Emitting Diodes. J. Sci. Adv. Mater. Devices 2021, 6, 372–378. [Google Scholar] [CrossRef]
- Ko, S.-J.; Choi, H.; Lee, W.; Kim, T.; Lee, B.R.; Jung, J.-W.; Jeong, J.-R.; Song, M.H.; Lee, J.C.; Woo, H.Y.; et al. Highly Efficient Plasmonic Organic Optoelectronic Devices Based on a Conducting Polymer Electrode Incorporated with Silver Nanoparticles. Energy Environ. Sci. 2013, 6, 1949–1955. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Ibrahim, M.M.; Abou Elfadl, F.I.; Hosni, H.M.; Shehata, E.M.; Deghiedy, N.M.; Balboul, M.R. PEDOT:PSS Incorporated Silver Nanoparticles Prepared by Gamma Radiation for the Application in Organic Solar Cells. J. Radiat. Res. Appl. Sci. 2015, 8, 166–172. [Google Scholar] [CrossRef]
- Morvillo, P.; De Girolamo Del Mauro, A.; Nenna, G.; Diana, R.; Ricciardi, R.; Minarini, C. ITO-Free Anode with Plasmonic Silver Nanoparticles for High Efficient Polymer Solar Cells. Energy Procedia 2014, 60, 13–22. [Google Scholar] [CrossRef]
- Ottonelli, M.; Zappia, S.; Demartini, A.; Alloisio, M. Chitosan-stabilized Noble Metal Nanoparticles: Study of Their Shape Evolution and Post- Functionalization Properties. Nanomaterials 2020, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.; Alloisio, M.; Darawish, R.; Dodero, A.; Vicini, S. Electrospun Composite Mats of Alginate with Embedded Silver Nanoparticles: Synthesis and Characterization. J. Therm. Anal. Calorim. 2019, 137, 767–778. [Google Scholar] [CrossRef]
- Alloisio, M.; Zappia, S.; Demartini, A.; Petrillo, G.; Ottonelli, M.; Thea, S.; Dellepiane, G.; Muniz-Miranda, M. Enhanced and Reproducible Photogeneration of Blue Poly(Pentacosadiacetylene) Chemisorbed onto Silver Nanoparticles: An Optimized Synthetic Protocol. Mater. Chem. Phys. 2014, 147, 293–303. [Google Scholar] [CrossRef]
- Moreno, K.J.; Moggio, I.; Arias, E.; Llarena, I.; Moya, S.E.; Ziolo, R.F.; Barrientos, H. Silver Nanoparticles Functionalized in Situ with the Conjugated Polymer (PEDOT:PSS). J. Nanosci. Nanotechnol. 2009, 9, 3987–3992. [Google Scholar] [CrossRef]
- Valdivia, J.C.; Pérez, A.; Rodríguez, G.; Hurtado, G.; Moggio, I.; Arias, E.; Zappia, S.; Destri, S.; Scavia, G.; Alloisio, M.; et al. Estudio Espectroscópico de Nanopartículas de Plata Pasivadas Con El Polímero Conjugado PEDOT:PSS Spectroscopic Study of Silver Nanoparticles Passivated with the Conjugated Polymer PEDOT:PSS. Superf. Vacío 2021, 34, 211101. [Google Scholar] [CrossRef]
- Glasser, A.; Cloutet, É.; Hadziioannou, G.; Kellay, H. Tuning the Rheology of Conducting Polymer Inks for Various Deposition Processes. Chem. Mater. 2019, 31, 6936–6944. [Google Scholar] [CrossRef]





| Sample | PEDOT:PSS Formulation | NaBH4/AgNO3 Molar Ratio | Ag Conc (mmol/L) |
|---|---|---|---|
| PP@AgNPs-1 | Orgacon | 9.9 | 40 |
| PP@AgNPs-2 | 4.9 | 40 | |
| PP@AgNPs-3 | 1.0 | 40 | |
| PP@AgNPs-4 | 0.1 | 40 | |
| PP@AgNPs-5 | Clevios | 0.1 | 40 |
| PP@AgNPs-6 | 0.1 | 2 | |
| PP@AgNPs-7 | 11.0 | 2 |
| Sample | Cluster Range Size (nm) | Cluster Average Size (nm) | Nanoparticle Average Size (Standard Deviation) (nm) | Nanoparticle Aspect Ratio (Standard Deviation) |
|---|---|---|---|---|
| PP@AgNPs-1 | 90–500 | - | 26 ± 1 (7) | 1.44 ± 0.06 (0.41) |
| PP@AgNPs-2 | 120–300 | 220 ± 10 | 32 ± 1 (9) | 1.39 ± 0.04 (0.31) |
| PP@AgNPs-3 | 60–600 | 200 ± 5 | 60 ± 1 (15) | 1.45 ± 0.04 (0.45) |
| PP@AgNPs-6 | 30–160 | 240 ± 20 | 21 ± 1 (5) | 1.31 ± 0.04 (0.25) |
| PP@AgNPs-7 | - | 70 ± 1 | 18 ± 1 (9) | 1.26 ± 0.03 (0.24) |
| Sample | η0 (Pa s) |
|---|---|
| PP@AgNPs-1 | 14.1 |
| PP@AgNPs-2 | 26.7 |
| PP@AgNPs-3 | 47.2 |
| PP@AgNPs-6 | 1.0 |
| PP@AgNPs-7 | 0.2 |
| Sample Label | NaBH4/AgNO3 Molar Ratio | Ag Conc (mmol/L) | Root Mean Square, RMS (nm) |
|---|---|---|---|
| PEDOT:PSS (Orgacon) | - | - | 1.2 |
| PP@AgNPs-1 | 9.9 | 40 | 33.0 |
| PP@AgNPs-2 | 4.9 | 40 | 4.3 |
| PP@AgNPs-3 | 1.0 | 40 | 5.3 |
| PP@AgNPs-4 | 0.1 | 40 | 7.7 |
| PP@AgNPs-5 | 0.1 | 40 | 4.4 |
| PP@AgNPs-6 | 0.1 | 2 | 1.3 |
| PP@AgNPs-7 | 11.0 | 2 | 8.1 |
| Electrode | Voc (Volts) | Jsc (mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|
| ITO 8–12 Ωsq | 0.60 | 5.13 | 0.55 | 1.70 |
| ITO 30–60 Ωsq | 0.53 | 3.16 | 0.30 | 0.51 |
| Orgacon/PP@AgNP-6 | 0.55 | 0.27 | 0.21 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappia, S.; Alloisio, M.; Valdivia, J.C.; Arias, E.; Moggio, I.; Scavia, G.; Destri, S. Silver Nanoparticle–PEDOT:PSS Composites as Water-Processable Anodes: Correlation between the Synthetic Parameters and the Optical/Morphological Properties. Polymers 2023, 15, 3675. https://doi.org/10.3390/polym15183675
Zappia S, Alloisio M, Valdivia JC, Arias E, Moggio I, Scavia G, Destri S. Silver Nanoparticle–PEDOT:PSS Composites as Water-Processable Anodes: Correlation between the Synthetic Parameters and the Optical/Morphological Properties. Polymers. 2023; 15(18):3675. https://doi.org/10.3390/polym15183675
Chicago/Turabian StyleZappia, Stefania, Marina Alloisio, Julio Cesar Valdivia, Eduardo Arias, Ivana Moggio, Guido Scavia, and Silvia Destri. 2023. "Silver Nanoparticle–PEDOT:PSS Composites as Water-Processable Anodes: Correlation between the Synthetic Parameters and the Optical/Morphological Properties" Polymers 15, no. 18: 3675. https://doi.org/10.3390/polym15183675





