Effect of Mineral Fertilizers and Pesticides Application on Bacterial Community and Antibiotic-Resistance Genes Distribution in Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Analysis of Soil Physicochemical Properties
2.3. Total DNA Isolation and Metagenomic Analysis of 16S rRNA Genes
2.4. Quantitative Measurement of Antibiotic Resistance Genes in the Studied Soils
2.5. Statistical Analysis
3. Results
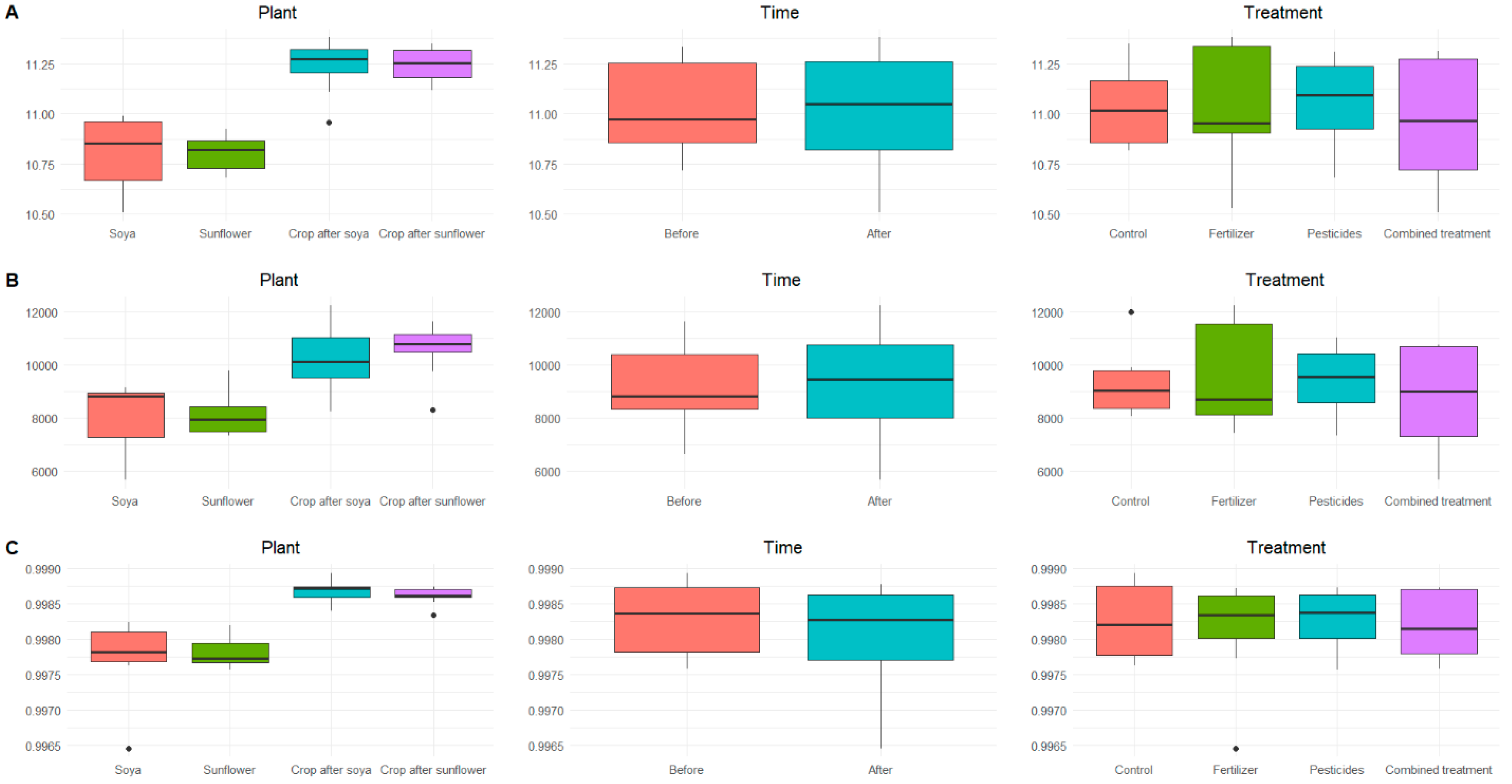
3.1. Soil Physicochemical Properties
3.2. Taxonomic Composition and Diversity of the Agricultural Soils Microbiome
3.3. Content of ARGs and Integrons in Agricultural Soils
3.4. Correlation Analysis of ARGs Content and Taxonomic Composition
3.5. Correlation between ARGs and Integrons in Agricultural Soils
4. Discussion
4.1. Effect of Agrochemical Treatments on the Bacterial Community of Agricultural Soils and Their Resistome
4.2. Relationship between Soil Bacterial Community and ARGs
4.3. The Importance of Horizontal Gene Transfer (HGT) in Formation of Agricultural Soil Resistomes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Plant- Protecting Agent | Trade Name | Composition | Application | Dose (L ha−1) | Treatment Method | Crop |
|---|---|---|---|---|---|---|
| Herbicides | Gardo Gold | 312.5 g L−1 c-metolachlor 187.5 g L−1 terbutylazine | SE | 4.0 | application to the soil before sowing | soya |
| 3.0 | sunflower | |||||
| Benito | 300 g L−1 bentazone | CC | 2.0 | spray to plant during vegetation | soya | |
| Reglon Super | 150 g L−1 diquat | WS | 2.0 | spray to plant before harvesting (desiccant) | sunflower | |
| Fungicides | Maxim | 25 g L−1 fludioxonil | SC | 5.0 | pre-sowing seed treatment (protectant) | sunflower |
| Optimo | 200 g L−1 pyraclostrobin | EC | 1.0 | spray to plant during growing season | sunflower | |
| Ceriax Plus | 66.6 g L−1 pyraclostrobin + 41.6 g L−1 fluxapyroxad + 41.6 g L−1 epoxiconazole | EC | 0.4 | spray to plant during growing season | winter wheat | |
| Insecticides | Cruiser | 350 g L−1 thiamethoxam | SC | 0.5 | pre-sowing seed treatment (seed dresser) | sunflower |
| Ampligo | 50 g L−1 lambda-cyhalothrin; 100 g L−1 chlorantraniliprole | MS | 0.2 | spray to plant during growing season | sunflower | |
| Fascord | 100 g L−1 alpha-cypermethrin | EC | 0.15 | spray to plant during growing season | winter wheat |
| № | Designation | Crop | Agrochemical Treatment | Sampling Time | Forecrop |
|---|---|---|---|---|---|
| Sampling before pesticide application | |||||
| 1 | Gc | soya | control | 14.06.2022 | – |
| 2 | Gf | soya | fertilizers | 14.06.2022 | – |
| 3 | Gp | soya | pesticides | 14.06.2022 | – |
| 4 | Gf + p | soya | fertilizers + pesticides | 14.06.2022 | – |
| 5 | Hc | sunflower | control | 14.06.2022 | – |
| 6 | Hf | sunflower | fertilizers | 14.06.2022 | – |
| 7 | Hp | sunflower | pesticides | 14.06.2022 | – |
| 8 | Hf + p | sunflower | fertilizers + pesticides | 14.06.2022 | – |
| 9 | T(g)c | winter wheat | control | 15.05.2023 | soya |
| 10 | T(g)f | winter wheat | fertilizers | 15.05.2023 | soya |
| 11 | T(g)p | winter wheat | pesticides | 15.05.2023 | soya |
| 12 | T(g)f + p | winter wheat | fertilizers + pesticides | 15.05.2023 | soya |
| 13 | T(h)c | winter wheat | control | 15.05.2023 | sunflower |
| 14 | T(h)f | winter wheat | fertilizers | 15.05.2023 | sunflower |
| 15 | T(h)p | winter wheat | pesticides | 15.05.2023 | sunflower |
| 16 | T(h)f + p | winter wheat | fertilizers + pesticides | 15.05.2023 | sunflower |
| Sampling after pesticide application | |||||
| 17 | Gc | soya | control | 07.07.2022 | – |
| 18 | Gf | soya | fertilizers | 07.07.2022 | – |
| 19 | Gp | soya | pesticides | 07.07.2022 | – |
| 20 | Gf + p | soya | fertilizers + pesticides | 07.07.2022 | – |
| 21 | Hc | sunflower | control | 22.09.2022 | – |
| 22 | Hf | sunflower | fertilizers | 22.09.2022 | – |
| 23 | Hp | sunflower | pesticides | 22.09.2022 | – |
| 24 | Hf + p | sunflower | fertilizers + pesticides | 22.09.2022 | – |
| 25 | T(g)c | winter wheat | control | 04.07.2023 | soya |
| 26 | T(g)f | winter wheat | fertilizers | 04.07.2023 | soya |
| 27 | T(g)p | winter wheat | pesticides | 04.07.2023 | soya |
| 28 | T(g)f + p | winter wheat | fertilizers + pesticides | 04.07.2023 | soya |
| 29 | T(h)c | winter wheat | control | 04.07.2023 | sunflower |
| 30 | T(h)f | winter wheat | fertilizers | 04.07.2023 | sunflower |
| 31 | T(h)p | winter wheat | pesticides | 04.07.2023 | sunflower |
| 32 | T(h)f + p | winter wheat | fertilizers + pesticides | 04.07.2023 | sunflower |
| Primer Name | Sequence, 5′-3′ | Amplicon Size, bp | PCR Conditions | Reference |
|---|---|---|---|---|
| 16S | f: GTGSTGCAYGGYTGTCGTCA r: ACGTCRTCCMCACCTTCCTC | 146 | 95 °C—3 min 95 °C—15 s 60 °C—60 s 72 °C—30 s 35 cycles | [65] |
| intI1 | f: GCCTTGATGTTACCCGAGAG r: GATCGGTCGAATGCGTGT | 196 | [66] | |
| intII2 | f: TGCTTTTCCCACCCTTACC r: GACGGCTACCCTCTGTTATCTC | 195 | ||
| intI3 | f: GCCACCACTTGTTTGAGGA r: GGATGTCTGTGCCTGCTTG | 138 | ||
| blaVIM-1 | f: ACTGTCGGATACTCACCACTC r: GTTATGGAGCAGCAACGATGT | 189 | 95 °C—3 min 95 °C—10 s 57 °C—35 s 72 °C—30 s 40 cycles | [67] |
| blaCTX-M | f: ACCAACGATATCGCGGTGAT r: ACATCGCGACGGCTTTCT | 101 | 95 °C—3 min 95 °C—15 s 58 °C—30 s 72 °C—30 s 40 cycles | [68] |
| mecA | f: GTGAAGATATACCAAGTGATT r: ATGCGCTATAGATTGAAAGGAT | 147 | [69] | |
| vanA | f: CATGGCAAGTCAGGTGAAGA r: CCACCGGCCTATCATCTTT | 187 | [70] | |
| vanB | f: AGACATTCCGGTCGAGGAAC r: GCTGTCAATTAGTGCGGGAA | 220 | 95 °C—3 min 95 °C—40 s 56,5 °C—40 s 72 °C—40 s 35 cycles | [71] |
| tetO | f: ATGGCATACAGGCACAGACC r: GGATGCTGCCCAACCTTTTG | 178 | 95 °C—3 min 95 °C—30 s 58 °C—40 s 72 °C—30 s 35 cycles | [72] |
| sul2 | f: TCCGGTGGAGGCCGGTATCTGG r: CGGGAATGCCATCTGCCTTGAG | 191 | [73] | |
| ermB | f: GCATTTAACGACGAAACTGGCT r: TGGTGAATTAAAGTGACACGAATGT | 123 | 95 °C—3 min 95 °C—10 s 59 °C—30 s 72 °C—45 s 40 cycles | [72] |
| mphA | f: AGTTCGTGGTGAACGACAAG r: AGTCGATCATCCCGCTGAC | 153 | 95 °C—3 min 95 °C—60 s 58 °C—60 s 72 °C—45 s 35 cycles | [74] |
| aadA2 | f: TAAGACGGGCTGATACTGG r: CATAGCGTTGCCTTGGTAG | 251 | 95 °C—3 min 95 °C—10 s 53 °C—30 s 72 °C—30 s 40 cycles | [75] |
| Crop | Sampling Time | Treatment | Solid Residue (% w/w) | NH4+–N (mg kg−1) | NO3−–N (mg kg−1) | P2O5 (mg kg−1) | K2O (mg kg−1) | pH | SOM (%) |
|---|---|---|---|---|---|---|---|---|---|
| Soya | before applying pesticides | control | 0.065 | 12.54 | 13.2 | 17.6 | 449.3 | 6.63 | 4.04 |
| fertilizers | 0.072 | 9.58 | 11 | 33.1 | 430.2 | 7.25 | 4.16 | ||
| pesticides | 0.0595 | 6.72 | 9.1 | 20 | 420.7 | 7.37 | 4.22 | ||
| combined treatment | 0.0705 | 8.96 | 21.9 | 27.8 | 468.5 | 7.04 | 4.23 | ||
| after applying pesticides | control | 0.08 | 9.07 | 13.2 | 21.1 | 497.1 | 6.9 | 4.16 | |
| fertilizers | 0.0768 | 9.58 | 10.5 | 22 | 411.1 | 6.74 | 4.02 | ||
| pesticides | 0.0735 | 8.34 | 6.3 | 14 | 344.2 | 7.26 | 4.06 | ||
| combined treatment | 0.1075 | 8.79 | 11.8 | 27.2 | 473.3 | 7.33 | 4.17 | ||
| Sunflower | before applying pesticides | control | 0.0603 | 6.53 | 5.9 | 17.5 | 411.1 | 6.87 | 4.08 |
| fertilizers | 0.065 | 7.62 | 7.6 | 23.1 | 430.2 | 6.66 | 4.1 | ||
| pesticides | 0.06 | 5.21 | 14.8 | 27.8 | 363.3 | 7.18 | 3.87 | ||
| combined treatment | 0.0793 | 5.04 | 19.5 | 46.7 | 272.8 | 7.17 | 3.91 | ||
| after applying pesticides | control | 0.0513 | 3.28 | 4.1 | 18.8 | 382.4 | 7.08 | 3.91 | |
| fertilizers | 0.055 | 4.07 | 3.4 | 24.3 | 439.8 | 7.17 | 4.16 | ||
| pesticides | 0.057 | 2.53 | 9.6 | 24.3 | 401.5 | 6.9 | 3.88 | ||
| combined treatment | 0.065 | 3.39 | 14.8 | 35.1 | 420.7 | 6.9 | 3.99 | ||
| Wheat grown after soya | before applying pesticides | control | 0.069 | 12.62 | 3.5 | 22.4 | 392 | 7.06 | 4.21 |
| fertilizers | 0.065 | 16.78 | 7.1 | 35.7 | 401.5 | 7.47 | 3.92 | ||
| pesticides | 0.059 | 12.12 | 6.2 | 26.4 | 392 | 7.23 | 4.2 | ||
| combined treatment | 0.051 | 14.2 | 7.2 | 28.1 | 411.1 | 7.25 | 4.09 | ||
| after applying pesticides | control | 0.072 | 6.79 | 3.4 | 17.8 | 439.8 | 7.25 | 4.14 | |
| fertilizers | 0.06 | 12.62 | 4.4 | 19.4 | 372.8 | 6.95 | 3.96 | ||
| pesticides | 0.0485 | 11.04 | 5.8 | 17.9 | 344.2 | 7.08 | 4.32 | ||
| combined treatment | 0.0465 | 10.29 | 5.9 | 17 | 420.7 | 7.81 | 3.98 | ||
| Wheat grown after sunflower | before applying pesticides | control | 0.0433 | 9.12 | 2.7 | 15.4 | 363.3 | 7.37 | 4.27 |
| fertilizers | 0.06 | 17.42 | 3.8 | 21.6 | 449.3 | 7.3 | 4.1 | ||
| pesticides | 0.0623 | 14.95 | 2.8 | 18.6 | 392 | 7.54 | 4.15 | ||
| combined treatment | 0.0693 | 15.2 | 4.6 | 23.7 | 449.3 | 7.41 | 4.2 | ||
| after applying pesticides | control | 0.048 | 7.12 | 2.2 | 17.1 | 344.2 | 7.43 | 4.05 | |
| fertilizers | 0.0593 | 12.45 | 3.1 | 16.5 | 382.4 | 8.02 | 3.93 | ||
| pesticides | 0.0628 | 12.37 | 1.4 | 14.8 | 369.8 | 7.31 | 4.06 | ||
| combined treatment | 0.0608 | 13.12 | 1.9 | 23.3 | 401.5 | 8.06 | 4.17 |
| Crop | Sampling Time | Treatment | α-Diversity Indices | ||
|---|---|---|---|---|---|
| Shannon | Chao1 | Simpson | |||
| Soya | before applying pesticides | control | 8.75817 | 686 | 0.99666 |
| fertilizers | 9.50461 | 1336 | 0.99763 | ||
| pesticides | 9.33305 | 1172 | 0.99742 | ||
| combined treatment | 9.51581 | 1250 | 0.99769 | ||
| after applying pesticides | control | 9.23950 | 1138 | 0.99677 | |
| fertilizers | 8.50721 | 698 | 0.99339 | ||
| pesticides | 9.39649 | 1234 | 0.99743 | ||
| combined treatment | 9.81576 | 1599 | 0.99757 | ||
| Sunflower | before applying pesticides | control | 8.74802 | 682 | 0.99656 |
| fertilizers | 8.60386 | 622 | 0.99615 | ||
| pesticides | 9.79218 | 1616 | 0.99791 | ||
| combined treatment | 9.59351 | 1413 | 0.99768 | ||
| after applying pesticides | control | 9.52641 | 1277 | 0.99767 | |
| fertilizers | 9.46248 | 1196 | 0.99761 | ||
| pesticides | 8.27541 | 431 | 0.99573 | ||
| combined treatment | 9.07144 | 926 | 0.99682 | ||
| Wheat grown after soya | before applying pesticides | control | 9.18216 | 851 | 0.99762 |
| fertilizers | 9.18137 | 838 | 0.99756 | ||
| pesticides | 8.55761 | 576 | 0.99643 | ||
| combined treatment | 8.56796 | 615 | 0.99627 | ||
| after applying pesticides | control | 8.77782 | 696 | 0.99684 | |
| fertilizers | 9.26384 | 979 | 0.99767 | ||
| pesticides | 8.62682 | 635 | 0.99646 | ||
| combined treatment | 8.74083 | 667 | 0.99676 | ||
| Wheat grown after sunflower | before applying pesticides | control | 9.24749 | 900 | 0.99774 |
| fertilizers | 8.70664 | 660 | 0.99678 | ||
| pesticides | 8.71410 | 660 | 0.99670 | ||
| combined treatment | 9.69050 | 1310 | 0.99808 | ||
| after applying pesticides | control | 8.83486 | 710 | 0.99701 | |
| fertilizers | 8.93322 | 782 | 0.99719 | ||
| pesticides | 8.69292 | 632 | 0.99672 | ||
| combined treatment | 8.98178 | 819 | 0.99718 | ||








References
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic Resistance Genes as Emerging Contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Pershina, E.; Valkonen, J.; Kurki, P.; Ivanova, E.; Chirak, E.; Korvigo, I.; Provorov, N.; Andronov, E. Comparative Analysis of Prokaryotic Communities Associated with Organic and Conventional Farming Systems. PLoS ONE 2015, 10, e0145072. [Google Scholar] [CrossRef]
- Silva, G.D.C.; Kitano, I.T.; Ribeiro, I.A.D.F.; Lacava, P.T. The Potential Use of Actinomycetes as Microbial Inoculants and Biopesticides in Agriculture. Front. Soil Sci. 2022, 2, 833181. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Das, N.; Kotoky, R.; Maurya, A.P.; Bhuyan, B.; Pandey, P. Paradigm Shift in Antibiotic-Resistome of Petroleum Hydrocarbon Contaminated Soil. Sci. Total Environ. 2021, 757, 143777. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Johnson, T.A.; Su, J.-Q.; Qiao, M.; Guo, G.-X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and Abundant Antibiotic Resistance Genes in Chinese Swine Farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Hu, H.-W.; Gou, M.; Wang, J.-T.; Chen, D.; He, J.-Z. Temporal Succession of Soil Antibiotic Resistance Genes Following Application of Swine, Cattle and Poultry Manures Spiked with or without Antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef]
- Wang, F.-H.; Qiao, M.; Su, J.-Q.; Chen, Z.; Zhou, X.; Zhu, Y.-G. High Throughput Profiling of Antibiotic Resistance Genes in Urban Park Soils with Reclaimed Water Irrigation. Environ. Sci. Technol. 2014, 48, 9079–9085. [Google Scholar] [CrossRef]
- Han, X.-M.; Hu, H.-W.; Shi, X.-Z.; Wang, J.-T.; Han, L.-L.; Chen, D.; He, J.-Z. Impacts of Reclaimed Water Irrigation on Soil Antibiotic Resistome in Urban Parks of Victoria, Australia. Environ. Pollut. 2016, 211, 48–57. [Google Scholar] [CrossRef]
- Xie, W.-Y.; McGrath, S.P.; Su, J.-Q.; Hirsch, P.R.; Clark, I.M.; Shen, Q.; Zhu, Y.-G.; Zhao, F.-J. Long-Term Impact of Field Applications of Sewage Sludge on Soil Antibiotic Resistome. Environ. Sci. Technol. 2016, 50, 12602–12611. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, L.; Zhu, S.; Lu, H.; Liu, W. Occurrence, Abundance, and Distribution of Sulfonamide and Tetracycline Resistance Genes in Agricultural Soils across China. Sci. Total Environ. 2017, 599–600, 1977–1983. [Google Scholar] [CrossRef]
- Jun, H.; Kurenbach, B.; Aitken, J.; Wasa, A.; Remus-Emsermann, M.N.P.; Godsoe, W.; Heinemann, J.A. Effects of Sub-Lethal Concentrations of Copper Ammonium Acetate, Pyrethrins and Atrazine on the Response of Escherichia coli to Antibiotics. F1000Research 2019, 8, 32. [Google Scholar] [CrossRef]
- Guo, A.; Pan, C.; Ma, J.; Bao, Y. Linkage of Antibiotic Resistance Genes, Associated Bacteria Communities and Metabolites in the Wheat Rhizosphere from Chlorpyrifos-Contaminated Soil. Sci. Total Environ. 2020, 741, 140457. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zhang, Q.; Long, Z.; Yu, Y.; Fang, H. Fungicides Enhanced the Abundance of Antibiotic Resistance Genes in Greenhouse Soil. Environ. Pollut. 2020, 259, 113877. [Google Scholar] [CrossRef]
- Rangasamy, K.; Athiappan, M.; Devarajan, N.; Parray, J.A. Emergence of Multi Drug Resistance among Soil Bacteria Exposing to Insecticides. Microb. Pathogen. 2017, 105, 153–165. [Google Scholar] [CrossRef]
- Kurenbach, B.; Marjoshi, D.; Amábile-Cuevas, C.F.; Ferguson, G.C.; Godsoe, W.; Gibson, P.; Heinemann, J.A. Sublethal Exposure to Commercial Formulations of the Herbicides Dicamba, 2,4-Dichlorophenoxyacetic Acid, and Glyphosate Cause Changes in Antibiotic Susceptibility in Escherichia coli and Salmonella enterica Serovar Typhimurium. mBio 2015, 6, e00009-15. [Google Scholar] [CrossRef]
- Sazykin, I.; Naumova, E.; Azhogina, T.; Klimova, M.; Karchava, S.; Khmelevtsova, L.; Chernyshenko, E.; Litsevich, A.; Khammami, M.; Sazykina, M. Glyphosate Effect on Biofilms Formation, Mutagenesis and Stress Response of E. coli. J. Hazard. Mater. 2024, 461, 132574. [Google Scholar] [CrossRef]
- Rangasamy, K.; Athiappan, M.; Devarajan, N.; Samykannu, G.; Parray, J.A.; Aruljothi, K.N.; Shameem, N.; Alqarawi, A.A.; Hashem, A.; Abd_Allah, E.F. Pesticide Degrading Natural Multidrug Resistance Bacterial Flora. Microb. Pathogen. 2018, 114, 304–310. [Google Scholar] [CrossRef]
- Egbe, C.C.; Oyetibo, G.O.; Ilori, M.O. Ecological Impact of Organochlorine Pesticides Consortium on Autochthonous Microbial Community in Agricultural Soil. Ecotoxicol. Environ. Saf. 2021, 207, 111319. [Google Scholar] [CrossRef]
- GOST 17.4.4.02-2017; Nature Protection. Soils. Methods for Sampling and Preparation of Soil for Chemical, Bacteriological, Helmintological Analysis. Standardinformrm: Moscow, Russia, 2018. (In Russian)
- Directive Document 2.1.7.730-799; Hygienic Assessment of Soil Quality in Populated Areas. Ministry of Health of the Russian Federation: Moscow, Russia, 1999. (In Russian)
- GOST 26489-85; Soils. Determination of Exchangeable Ammonium by CINAO Method. Standards Publishing House: Moscow, Russia, 1985. (In Russian)
- GOST 26951-86; Soils. Determination of Nitrates by Ionometric Method. Standards Publishing House: Moscow, Russia, 1986. (In Russian)
- GOST 26205-91; Soils. Determination of Mobile Compounds of Phosphorus and Potassium by Machigin Method Modified by CINAO. Standards Publishing House: Moscow, Russia, 1992. (In Russian)
- GOST 26213-2021; Soils. Methods for Determination of Organic Matter. Russian Standardization Institute: Moscow, Russia, 2021. (In Russian)
- GOST 26423-85; Soils. Methods for Determination of Specific Electric Conductivity, pH and Solid Residue of Water Extract. Standartinform: Moscow, Russia, 2011. (In Russian)
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Moreira-Grez, B.; Tam, K.; Cross, A.T.; Yong, J.W.H.; Kumaresan, D.; Nevill, P.; Farrell, M.; Whiteley, A.S. The Bacterial Microbiome Associated With Arid Biocrusts and the Biogeochemical Influence of Biocrusts Upon the Underlying Soil. Front. Microbiol. 2019, 10, 2143. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Niftrik, L.V.; Strous, M.; Kartal, B.; Keltjens, J.T.; Op Den Camp, H.J.M. Biochemistry and Molecular Biology of Anammox Bacteria. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 65–84. [Google Scholar] [CrossRef]
- Pershina, E.V.; Ivanova, E.A.; Korvigo, I.O.; Chirak, E.L.; Sergaliev, N.H.; Abakumov, E.V.; Provorov, N.A.; Andronov, E.E. Investigation of the Core Microbiome in Main Soil Types from the East European Plain. Sci. Total Environ. 2018, 631–632, 1421–1430. [Google Scholar] [CrossRef]
- Richaume, A.; Smit, E.; Faurie, G.; Elsas, J.D. Influence of Soil Type on the Transfer of Plasmid RP4p from Pseudomonas fluorescens to Introduced Recipient and to Indigenous Bacteria. FEMS Microb. Lett. 1992, 101, 281–291. [Google Scholar] [CrossRef]
- Lu, X.-M.; Lu, L.-B.; Lin, Y.-H.; Chen, Z.-Y.; Chen, J.-H. Exploring the Interaction between Agronomic Practices and Soil Characteristics on the Presence of Antibiotic Resistance Genes in Soil. Appl. Soil Ecol. 2023, 187, 104837. [Google Scholar] [CrossRef]
- Wang, C.; Henry, H.A.L.; Miao, X.; Shi, B.; Song, Y.; Liang, Q.; Sun, W. Seasonal Variation Modifies the Spatial Patterns of Soil Microbial Community Structure and Enzyme Activity in a Meadow Steppe. Appl. Soil Ecol. 2023, 182, 104686. [Google Scholar] [CrossRef]
- Lal, R.; Pandey, G.; Sharma, P.; Kumari, K.; Malhotra, S.; Pandey, R.; Raina, V.; Kohler, H.-P.E.; Holliger, C.; Jackson, C.; et al. Biochemistry of Microbial Degradation of Hexachlorocyclohexane and Prospects for Bioremediation. Microbiol. Mol. Biol. Rev. 2010, 74, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.C.; Rayu, S.; Nielsen, U.N.; Lai, K.; Ijaz, A.; Nazaries, L.; Singh, B.K. Metagenomic Functional Potential Predicts Degradation Rates of a Model Organophosphorus Xenobiotic in Pesticide Contaminated Soils. Front. Microbiol. 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Khmelevtsova, L.; Konstantinova, E.; Karchava, S.; Klimova, M.; Azhogina, T.; Polienko, E.; Khammami, M.; Sazykin, I.; Sazykina, M. Influence of Pesticides and Mineral Fertilizers on the Bacterial Community of Arable Soils under Pea and Chickpea Crops. Agronomy 2023, 13, 750. [Google Scholar] [CrossRef]
- Sotelo, J.P.; Paletti Rovey, M.F.; Carezzano, M.E.; Moliva, M.V.; Oliva, M.D.L.M. Characterization of Pseudomonas Syringae Strains Associated with Soybean Bacterial Blight and in Vitro Inhibitory Effect of Oregano and Thyme Essential Oils. Physiol. Mol. Plant Pathol. 2023, 128, 102133. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Murali, M.; Shilpa, N.; Amruthesh, K.N.; Gafur, A.; Antonius, S.; Sayyed, R.Z. Harnessing Abiotic Elicitors to Bolster Plant’s Resistance against Bacterial Pathogens. Plant Stress. 2024, 11, 100371. [Google Scholar] [CrossRef]
- Lin, H.; Sun, W.; Zhang, Z.; Chapman, S.J.; Freitag, T.E.; Fu, J.; Zhang, X.; Ma, J. Effects of Manure and Mineral Fertilization Strategies on Soil Antibiotic Resistance Gene Levels and Microbial Community in a Paddy–Upland Rotation System. Environ. Pollut. 2016, 211, 332–337. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Z.; Liu, S.; Sheng, Y.; Waigi, M.G.; Hu, X.; Qin, C.; Ling, W. Genotoxicity of Organic Contaminants in the Soil: A Review Based on Bibliometric Analysis and Methodological Progress. Chemosphere 2023, 313, 137318. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, W.; Chen, S.; Dong, W.; Qiao, M.; Hu, C.; Liu, B. Fifteen-Year Application of Manure and Chemical Fertilizers Differently Impacts Soil ARGs and Microbial Community Structure. Front. Microbiol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Yuan, S.-T.; Xu, M.-G.; Yang, X.-P.; Shen, Q.-R.; Zhang, W.-W.; Su, J.-Q.; Zhao, F.-J. Long-Term Effects of Manure and Chemical Fertilizers on Soil Antibiotic Resistome. Soil Biol. Biochem. 2018, 122, 111–119. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, T.; Tang, J.; Ling, H.; Wu, X. Key Taxa and Mobilome-Mediated Responses Co-Reshape the Soil Antibiotic Resistome under Dazomet Fumigation Stress. Environ. Internat. 2023, 182, 108318. [Google Scholar] [CrossRef] [PubMed]
- Willms, I.M.; Kamran, A.; Aßmann, N.F.; Krone, D.; Bolz, S.H.; Fiedler, F.; Nacke, H. Discovery of Novel Antibiotic Resistance Determinants in Forest and Grassland Soil Metagenomes. Front. Microbiol. 2019, 10, 460. [Google Scholar] [CrossRef]
- Wu, J.; Guo, S.; Lin, H.; Li, K.; Li, Z.; Wang, J.; Gaze, W.H.; Zou, J. Uncovering the Prevalence and Drivers of Antibiotic Resistance Genes in Soils across Different Land-Use Types. J. Environ. Manag. 2023, 344, 118920. [Google Scholar] [CrossRef]
- Song, M.; Song, D.; Jiang, L.; Zhang, D.; Sun, Y.; Chen, G.; Xu, H.; Mei, W.; Li, Y.; Luo, C.; et al. Large-Scale Biogeographical Patterns of Antibiotic Resistome in the Forest Soils across China. J. Hazard. Mater. 2021, 403, 123990. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Chen, Q.-L.; Zhu, D.; An, X.-L.; Yang, X.-R.; Su, J.-Q.; Qiao, M.; Zhu, Y.-G. Spatial and Temporal Distribution of Antibiotic Resistomes in a Peri-Urban Area Is Associated Significantly with Anthropogenic Activities. Environ. Pollut. 2018, 235, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the Wild: Antibiotic Resistance Genes in Natural Environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K.J.; Patel, S.; Gibson, M.K.; Lauber, C.L.; Knight, R.; Fierer, N.; Dantas, G. Bacterial Phylogeny Structures Soil Resistomes across Habitats. Nature 2014, 509, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qian, X.; Gu, J.; Wang, X.-J.; Duan, M.-L. Mechanism and Effect of Temperature on Variations in Antibiotic Resistance Genes during Anaerobic Digestion of Dairy Manure. Sci. Rep. 2016, 6, 30237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-Years of Fertilization on Bacterial Communities in an Intensively Cultivated Black Soil in Northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic Resistance Gene Spread Due to Manure Application on Agricultural Fields. Cur. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Huang, D.; Zhang, J.; Yao, Y.; Zhang, G.; Ju, F.; Xu, B.; Wang, M. Reduction of Antibiotic Resistance Genes (ARGs) in Swine Manure-Fertilized Soil via Fermentation Broth from Fruit and Vegetable Waste. Environ. Res. 2022, 214, 113835. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Li, J.; Li, J.; Ma, Y.; Chen, D.; He, J. Field-based Evidence for Copper Contamination Induced Changes of Antibiotic Resistance in Agricultural Soils. Environ. Microbiol. 2016, 18, 3896–3909. [Google Scholar] [CrossRef]
- Song, T.; Zhu, C.; Li, B.; Yan, M.; Li, H. Manure Application Led to Higher Antibiotic Resistance Risk in Red Soil Compared with Black Soil and Fluvo-Aquic Soil. J. Hazard. Mater. Adv. 2023, 9, 100209. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Singh, B.K.; Liu, Y.; Chen, Y.; Zhang, Y.; He, J. Diversity of Herbaceous Plants and Bacterial Communities Regulates Soil Resistome across Forest Biomes. Environ. Microbiol. 2018, 20, 3186–3200. [Google Scholar] [CrossRef]
- Cerqueira, F.; Matamoros, V.; Bayona, J.M.; Berendonk, T.U.; Elsinga, G.; Hornstra, L.M.; Piña, B. Antibiotic Resistance Gene Distribution in Agricultural Fields and Crops. A Soil-to-Food Analysis. Environ. Res. 2019, 177, 108608. [Google Scholar] [CrossRef]
- Du, S.; Shen, J.-P.; Hu, H.-W.; Wang, J.-T.; Han, L.-L.; Sheng, R.; Wei, W.-X.; Fang, Y.-T.; Zhu, Y.-G.; Zhang, L.-M.; et al. Large-Scale Patterns of Soil Antibiotic Resistome in Chinese Croplands. Sci. Total Environ. 2020, 712, 136418. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.-G. Using the Class 1 Integron-Integrase Gene as a Proxy for Anthropogenic Pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- An, X.-L.; Abass, O.K.; Zhao, C.-X.; Xu, M.-R.; Pan, T.; Pu, Q.; Liao, H.; Li, H.; Zhu, Y.-G.; Su, J.-Q. Nanopore Sequencing Analysis of Integron Gene Cassettes in Sewages and Soils. Sci. Total Environ. 2022, 817, 152766. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, Y.; Shi, X.; Yan, M.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of Zn and Oxytetracycline on Mobile Genetic Elements, Antibiotic Resistance Genes, and Microbial Community Evolution in Soil. Environ. Pollut. 2024, 341, 122609. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Zhang, Z.; Zhou, S.; Jin, M.; Zhu, D.; Yang, X.; Qian, H.; Lu, T. Plants Select Antibiotic Resistome in Rhizosphere in Early Stage. Sci. Total Environ. 2023, 858, 159847. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Baluja, M.; Frigon, D.; Abouelnaga, M.; Jobling, K.; Romalde, J.L.; Gomez Lopez, M.; Graham, D.W. Dynamics of Integron Structures across a Wastewater Network–Implications to Resistance Gene Transfer. Water Res. 2021, 206, 117720. [Google Scholar] [CrossRef]
- Tan, L.; Li, L.; Ashbolt, N.; Wang, X.; Cui, Y.; Zhu, X.; Xu, Y.; Yang, Y.; Mao, D.; Luo, Y. Arctic Antibiotic Resistance Gene Contamination, a Result of Anthropogenic Activities and Natural Origin. Sci. Total Environ. 2018, 621, 1176–1184. [Google Scholar] [CrossRef]
- Guo, X.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of Antibiotic Resistance Genes under Extremely High-Level Antibiotic Selection Pressures in Pharmaceutical Wastewater Treatment Plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef]
- Zhang, K.; McClure, J.-A.; Elsayed, S.; Louie, T.; Conly, J.M. Novel Multiplex PCR Assay for Characterization and Concomitant Subtyping of Staphylococcal Cassette Chromosome Mec Types I to V in Methicillin-Resistant Staphylococcus Aureus. J. Clin. Microbiol. 2005, 43, 5026–5033. [Google Scholar] [CrossRef]
- Lekunberri, I.; Villagrasa, M.; Balcázar, J.L.; Borrego, C.M. Contribution of Bacteriophage and Plasmid DNA to the Mobilization of Antibiotic Resistance Genes in a River Receiving Treated Wastewater Discharges. Sci. Total Environ. 2017, 601–602, 206–209. [Google Scholar] [CrossRef]
- Akanbi, O.E.; Njom, H.A.; Fri, J.; Otigbu, A.C.; Clarke, A.M. Antimicrobial Susceptibility of Staphylococcus Aureus Isolated from Recreational Waters and Beach Sand in Eastern Cape Province of South Africa. IJERPH 2017, 14, 1001. [Google Scholar] [CrossRef]
- Azhogina, T.; Sazykina, M.; Konstantinova, E.; Khmelevtsova, L.; Minkina, T.; Antonenko, E.; Sushkova, S.; Khammami, M.; Mandzhieva, S.; Sazykin, I. Bioaccessible PAH Influence on Distribution of Antibiotic Resistance Genes and Soil Toxicity of Different Types of Land Use. Environ. Sci. Pollut. Res. 2022, 30, 12695–12713. [Google Scholar] [CrossRef]
- Wang, M.; Liu, P.; Xiong, W.; Zhou, Q.; Wangxiao, J.; Zeng, Z.; Sun, Y. Fate of Potential Indicator Antimicrobial Resistance Genes (ARGs) and Bacterial Community Diversity in Simulated Manure-Soil Microcosms. Ecotoxicol. Environ. Saf. 2018, 147, 817–823. [Google Scholar] [CrossRef]
- Szczepanowski, R.; Linke, B.; Krahn, I.; Gartemann, K.-H.; Gützkow, T.; Eichler, W.; Pühler, A.; Schlüter, A. Detection of 140 Clinically Relevant Antibiotic-Resistance Genes in the Plasmid Metagenome of Wastewater Treatment Plant Bacteria Showing Reduced Susceptibility to Selected Antibiotics. Microbiology 2009, 155, 2306–2319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Zhao, Z.; Chen, J.; Lu, H.; Liu, G.; Zhou, J.; Guan, X. PAHs Accelerate the Propagation of Antibiotic Resistance Genes in Coastal Water Microbial Community. Environ. Pollut. 2017, 231, 1145–1152. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khmelevtsova, L.; Azhogina, T.; Karchava, S.; Klimova, M.; Polienko, E.; Litsevich, A.; Chernyshenko, E.; Khammami, M.; Sazykin, I.; Sazykina, M. Effect of Mineral Fertilizers and Pesticides Application on Bacterial Community and Antibiotic-Resistance Genes Distribution in Agricultural Soils. Agronomy 2024, 14, 1021. https://doi.org/10.3390/agronomy14051021
Khmelevtsova L, Azhogina T, Karchava S, Klimova M, Polienko E, Litsevich A, Chernyshenko E, Khammami M, Sazykin I, Sazykina M. Effect of Mineral Fertilizers and Pesticides Application on Bacterial Community and Antibiotic-Resistance Genes Distribution in Agricultural Soils. Agronomy. 2024; 14(5):1021. https://doi.org/10.3390/agronomy14051021
Chicago/Turabian StyleKhmelevtsova, Ludmila, Tatiana Azhogina, Shorena Karchava, Maria Klimova, Elena Polienko, Alla Litsevich, Elena Chernyshenko, Margarita Khammami, Ivan Sazykin, and Marina Sazykina. 2024. "Effect of Mineral Fertilizers and Pesticides Application on Bacterial Community and Antibiotic-Resistance Genes Distribution in Agricultural Soils" Agronomy 14, no. 5: 1021. https://doi.org/10.3390/agronomy14051021
APA StyleKhmelevtsova, L., Azhogina, T., Karchava, S., Klimova, M., Polienko, E., Litsevich, A., Chernyshenko, E., Khammami, M., Sazykin, I., & Sazykina, M. (2024). Effect of Mineral Fertilizers and Pesticides Application on Bacterial Community and Antibiotic-Resistance Genes Distribution in Agricultural Soils. Agronomy, 14(5), 1021. https://doi.org/10.3390/agronomy14051021






