Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells
Abstract
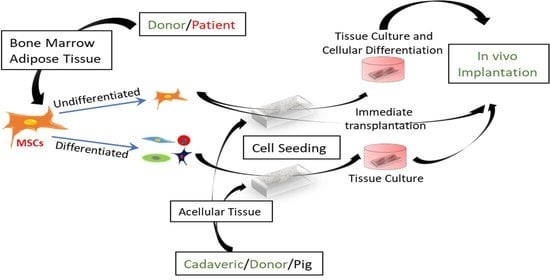
:1. Introduction
2. Role of MSCs in the Natural-Derived Scaffolds Mediated Regeneration
2.1. Differentiation of Functional Cells
2.2. Regulation of Immune Action
2.3. Increase of Angiogenesis
3. Recellularization of Scaffolds with MSCs
3.1. Methods of Recellularization
3.2. The Factors That Are Responsible for Recellularization Efficacy
3.2.1. The Anatomical Structure and Pathological Conditions of Scaffold Sources
3.2.2. Method of Decellularization
3.2.3. The Surface Modification of Scaffolds
3.2.4. Source and Abundance of MSCs
3.2.5. The Microenvironment of Cell Culture
3.3. Limitations of MSCs for Recellularization of Acellular Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukumitsu, K.; Yagi, H.; Soto-Gutierrez, A. Bioengineering in organ transplantation: Targeting the liver. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; Volume 43, pp. 2137–2138. [Google Scholar]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Massé, S.; Magtibay, K.; Kawajiri, H.; et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.-F.; Li, Y.-B.; Gao, X.-J.; Zhang, H.-Y.; Lin, S.; Zhu, Y.-Y. Efficacy and safety of autologous stem cell transplantation for decompensated liver cirrhosis: A retrospective cohort study. World J. Stem Cells 2018, 10, 138. [Google Scholar] [CrossRef]
- Rouchi, A.H.; Mahdavi-Mazdeh, M. Regenerative medicine in organ and tissue transplantation: Shortly and practically achievable? Int. J. Organ Transplant. Med. 2015, 6, 93. [Google Scholar]
- Lesman, A.; Habib, M.; Caspi, O.; Gepstein, A.; Arbel, G.; Levenberg, S.; Gepstein, L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng. Part A 2010, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Serafini, F.; Pierdomenico, L.; Simeone, P.; Bologna, G.; Ercolino, E.; Di Silvestre, S.; Guarnieri, S.; Canosa, C.; Impicciatore, G.G.; et al. Human Mesenchymal Stem Cells Reendothelialize Porcine Heart Valve Scaffolds: Novel Perspectives in Heart Valve Tissue Engineering. BioRes. Open Access 2015, 4, 288–297. [Google Scholar] [CrossRef]
- Chen, Y.L.; Sun, C.K.; Tsai, T.H.; Chang, L.T.; Leu, S.; Zhen, Y.Y.; Sheu, J.J.; Chua, S.; Yeh, K.H.; Lu, H.I.; et al. Adipose-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffolds promote angiogenesis, preserve heart function, and reduce left ventricular remodeling in rat acute myocardial infarction. Am. J. Transl. Res. 2015, 7, 781–803. [Google Scholar] [PubMed]
- Ko, I.K.; Peng, L.; Peloso, A.; Smith, C.J.; Dhal, A.; Deegan, D.B.; Zimmerman, C.; Clouse, C.; Zhao, W.; Shupe, T.D.; et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials 2015, 40, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Ahn, J.; Kang, H.K.; Kim, M.-S.; Kim, N.-G.; Kook, M.G.; Choi, S.W.; Jeon, N.L.; Woo, H.M.; Kang, K.S. Development of highly functional bioengineered human liver with perfusable vasculature. Biomaterials 2021, 265, 120417. [Google Scholar] [CrossRef]
- Sabetkish, S.; Sabektish, N.; Ekhtiari, M.; Jobani, B.M.; Kajbafzadeh, A.M. Decellularization and Recellularization of Rabbit Kidney Using Adipose-Derived Mesenchymal Stem Cells for Renal. Regen. Eng. Transl. Med. 2020, 6, 433–441. [Google Scholar] [CrossRef]
- Xue, A.; Niu, G.; Chen, Y.; Li, K.; Xiao, Z.; Luan, Y.; Sun, C.; Xie, X.; Zhang, D.; Du, X.; et al. Recellularization of well-preserved decellularized kidney scaffold using adipose tissue-derived stem cells. J. Biomed. Mater. Res. Part A 2018, 106, 805–814. [Google Scholar] [CrossRef]
- Doi, R.; Tsuchiya, T.; Mitsutake, N.; Nishimura, S.; Matsuu-Matsuyama, M.; Nakazawa, Y.; Ogi, T.; Akita, S.; Yukawa, H.; Baba, Y.; et al. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrika, K.U.; Tripathi, R.; Kameshwari, Y.; Rangaraj, N.; Kumar, J.M.; Singh, S. Refunctionalization of Decellularized Organ Scaffold of Pancreas by Recellularization: Whole Organ Regeneration into Functional Pancreas. Tissue Eng. Regen. Med. 2020, 18, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Pineda Molina, C.; Lee, Y.C.; Badylak, S.F. Chapter 38-Pancreas whole organ engineering. In Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas; Orlando, G., Piemonti, L., Ricordi, C., Stratta, R.J., Gruessner, R.W.G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 527–536. [Google Scholar]
- Ibsirlioglu, T.; Elçin, A.E.; Elçin, Y.M. Decellularized biological scaffold and stem cells from autologous human adipose tissue for cartilage tissue engineering. Methods 2020, 171, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Scholl, F.G.; Boucek, M.M.; Chan, K.-C.; Valdes-Cruz, L.; Perryman, R. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: Human applications in congenital heart disease. World J. Pediatric Congenit. Heart Surg. 2010, 1, 132–136. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Y.; Wang, Z.; Wang, W.; Schmull, S.; Dong, J.; Xue, S.; Imboden, H.; Li, J. Human heart valve-derived scaffold improves cardiac repair in a murine model of myocardial infarction. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waltz, E. When pig organs will fly. Nat. Biotechnol. 2017, 35, 1133–1138. [Google Scholar] [CrossRef]
- Mazza, G.; Rombouts, K.; Hall, A.R.; Urbani, L.; Luong, T.V.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Struecker, B.; Hillebrandt, K.H.; Voitl, R.; Butter, A.; Schmuck, R.B.; Reutzel-Selke, A.; Geisel, D.; Joehrens, K.; Pickerodt, P.A.; Raschzok, N.; et al. Porcine liver decellularization under oscillating pressure conditions: A technical refinement to improve the homogeneity of the decellularization process. Tissue Eng. Part C Methods 2015, 21, 303–313. [Google Scholar] [CrossRef]
- Ahmed, E.; Saleh, T.; Yu, L.; Kwak, H.-H.; Kim, B.-M.; Park, K.-M.; Lee, Y.-S.; Kang, B.-J.; Choi, K.-Y.; Kang, K.-S.; et al. Micro and ultrastructural changes monitoring during decellularization for the generation of a biocompatible liver. J. Biosci. Bioeng. 2019, 128, 218–225. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Butter, A.; Aliyev, K.; Hillebrandt, K.H.; Raschzok, N.; Kluge, M.; Seiffert, N.; Tang, P.; Napierala, H.; Muhamma, A.I.; Reutzel-Selke, A.; et al. Evolution of graft morphology and function after recellularization of decellularized rat livers. J. Tissue Eng. Regen. Med. 2018, 12, e807–e816. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, M.; Duchesneau, P.; Taniguchi, D.; Waddell, T.K.; Karoubi, G. Negative pressure cell delivery augments recellularization of decellularized lungs. Tissue Eng. Part C Methods 2021, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bonandrini, B.; Figliuzzi, M.; Papadimou, E.; Morigi, M.; Perico, N.; Casiraghi, F.; Dipl, C.; Sangalli, F.; Conti, S.; Benigni, A.; et al. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng. Part A 2014, 20, 1486–1498. [Google Scholar] [CrossRef] [Green Version]
- Daly, A.B.; Wallis, J.M.; Borg, Z.D.; Bonvillain, R.W.; Deng, B.; Ballif, B.A.; Jaworski, D.M.; Allen, G.B.; Weiss, D.J. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng. Part A 2012, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Bilodeau, C.; Goltsis, O.; Rogers, I.M.; Post, M. Limitations of recellularized biological scaffolds for human transplantation. J. Tissue Eng. Regen. Med. 2020, 14, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Bonvillain, R.W.; Danchuk, S.; Sullivan, D.E.; Betancourt, A.M.; Semon, J.A.; Eagle, M.E.; Mayeux, J.P.; Gregory, A.N.; Wang, G.; Townley, I.K.; et al. A nonhuman primate model of lung regeneration: Detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng. Part A 2012, 18, 2437–2452. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.B.; Benkusky, N.A.; Sen, B.; Rubin, J.; Pike, J.W. Epigenetic Plasticity Drives Adipogenic and Osteogenic Differentiation of Marrow-derived Mesenchymal Stem Cells. J. Biol. Chem. 2016, 291, 17829–17847. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wąsiatycz, G.; Antosik, P.; et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. [Google Scholar] [CrossRef]
- El Omar, R.; Beroud, J.; Stoltz, J.-F.; Menu, P.; Velot, E.; Decot, V. Umbilical cord mesenchymal stem cells: The new gold standard for mesenchymal stem cell-based therapies? Tissue Eng. Part B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Darmayanti, S.; Triana, R.; Chouw, A.; Dewi, N.M. Is Stem Cell a Curer or an Obstruction? Mol. Cell. Biomed. Sci. 2017, 1, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Tovar, I.; Guerrero, R.; López-Peñalver, J.J.; Expósito, J.; Ruiz de Almodóvar, J.M. Rationale for the use of radiation-activated mesenchymal stromal/stem cells in acute respiratory distress syndrome. Cells 2020, 9, 2015. [Google Scholar] [CrossRef]
- Hasan, A.; Deeb, G.; Rahal, R.; Atwi, K.; Mondello, S.; Marei, H.E.; Gali, A.; Sleiman, E. Mesenchymal stem cells in the treatment of traumatic brain injury. Front. Neurol. 2017, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef]
- Li, H.; Zuo, S.; He, Z.; Yang, Y.; Pasha, Z.; Wang, Y.; Xu, M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1772–H1781. [Google Scholar] [CrossRef] [Green Version]
- Duffy, G.P.; Ahsan, T.; O’Brien, T.; Barry, F.; Nerem, R.M. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng. Part A 2009, 15, 2459–2470. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Ling, X.; Hu, G.; Zhu, Q.; Zhang, J.; Li, Q.; Zhao, B.; Wang, Y.; Deng, Z. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res. Ther. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Sabry, D.; Mohamed, A.; Monir, M.; Ibrahim, H.A. The Effect of Mesenchymal Stem Cells Derived Microvesicles on the Treatment of Experimental CCL4 Induced Liver Fibrosis in Rats. Int. J. Stem Cells 2019, 12, 400. [Google Scholar] [CrossRef] [Green Version]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling self-renewal and differentiation of stem cells via mechanical cues. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uygun, B.E.; Price, G.; Saeidi, N.; Izamis, M.-L.; Berendsen, T.; Yarmush, M.; Uygun, K. Decellularization and recellularization of whole livers. J. Vis. Exp. 2011, 48, e2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinkovic, M.; Block, T.J.; Rakian, R.; Li, Q.; Wang, E.; Reilly, M.A.; Dean, D.D.; Chen, X.D. One size does not fit all: Developing a cell-specific niche for in vitro study of cell behavior. Matrix Biol. 2016, 52, 426–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol. Bioeng. 2006, 93, 1152–1163. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Rothrauff, B.B.; Lin, H.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 2013, 34, 9295–9306. [Google Scholar] [CrossRef] [Green Version]
- Vertelov, G.; Gutierrez, E.; Lee, S.-A.; Ronan, E.; Groisman, A.; Tkachenko, E. Rigidity of silicone substrates controls cell spreading and stem cell differentiation. Sci. Rep. 2016, 6, 33411. [Google Scholar] [CrossRef] [Green Version]
- Witkowska-Zimny, M.; Walenko, K.; Wałkiewicz, A.E.; Pojda, Z.; Przybylski, J.; Lewandowska-Szumieł, M. Effect of substrate stiffness on differentiation of umbilical cord stem cells. Acta Biochim. Pol. 2012, 59, 2. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Zacharek, A.; Chen, J.; Li, A.; Cui, X.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691. [Google Scholar] [CrossRef] [Green Version]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 2012, 227, 88–97. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, W.; Yang, L.; Liang, C.; Lin, Y.; Wei, X.; Xu, B. In vivo immunogenicity of bovine bone removed by a novel decellularization protocol based on supercritical carbon dioxide. Artif. Cells Nanomed. Biotechnol. 2018, 46, 334–344. [Google Scholar]
- Wiles, K.; Fishman, J.M.; De Coppi, P.; Birchall, M.A. The host immune response to tissue-engineered organs: Current problems and future directions. Tissue Eng. Part B Rev. 2016, 22, 208–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haykal, S.; Zhou, Y.; Marcus, P.; Salna, M.; Machuca, T.; Hofer, S.O.; Waddell, K.T. The effect of decellularization of tracheal allografts on leukocyte infiltration and of recellularization on regulatory T cell recruitment. Biomaterials 2013, 34, 5821–5832. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wan, M.; Lyon, C.J.; Hu, T.Y. Nanomedicine therapies modulating Macrophage Dysfunction: A potential strategy to attenuate Cytokine Storms in severe infections. Theranostics 2020, 10, 9591. [Google Scholar] [CrossRef]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, 21, 7061. [Google Scholar]
- Chao, Y.H.; Wu, H.P.; Wu, K.H.; Tsai, Y.G.; Peng, C.T.; Lin, K.C.; Chao, W.R.; Lee, M.S.; Fu, Y.C. An increase in CD3+ CD4+ CD25+ regulatory T cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis. PLoS ONE 2014, 9, e110338. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, H.; Yang, B.; Sun, Y.; Huo, R. Three-dimensional graphene foams loaded with bone marrow derived mesenchymal stem cells promote skin wound healing with reduced scarring. Mater. Sci. Eng. C 2015, 57, 181–188. [Google Scholar] [CrossRef]
- Boomsma, R.A.; Geenen, D.L. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS ONE 2012, 7, e35685. [Google Scholar] [CrossRef] [Green Version]
- Lachmann, N. Therapeutic angiogenesis for peripheral artery disease: Stem cell therapy. Vasa 2007, 36, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Wang, W.E.; Zeng, C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016, 2016, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Bronshtein, T.; Boey, F.Y.C.; Venkatraman, S.S.; Machluf, M. Chemical modification of porcine acelluar extracellular matrix for cardiovascular tissue regeneration. Int. Conf. Biol. Life Sci. 2012, 40, 159–163. [Google Scholar]
- Han, T.T.Y.; Flynn, L.E. Perfusion bioreactor culture of human adipose-derived stromal cells on decellularized adipose tissue scaffolds enhances in vivo adipose tissue regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1827–1840. [Google Scholar] [CrossRef]
- Sarig, U.; Nguyen, E.B.-V.; Wang, Y.; Ting, S.; Bronshtein, T.; Sarig, H.; Dahan, N.; Gvirtz, M.; Reuveny, S.; Oh, S.K.; et al. Pushing the envelope in tissue engineering: Ex vivo production of thick vascularized cardiac extracellular matrix constructs. Tissue Eng. Part A 2015, 21, 1507–1519. [Google Scholar] [CrossRef] [Green Version]
- Muniswami, D.M.; Reddy, L.V.K.; Amirtham, S.M.; Babu, S.; Raj, A.N.; Sen, D.; Manivasagam, G. Endothelial progenitor/stem cells in engineered vessels for vascular transplantation. J. Mater. Sci. Mater. Med. 2020, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khorramirouz, R.; Go, J.L.; Noble, C.; Morse, D.; Lerman, A.; Young, M.D. In vivo response of acellular porcine pericardial for tissue engineered transcatheter aortic valves. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Vincentelli, A.; Wautot, F.; Juthier, F.; Fouquet, O.; Corseaux, D.; Marechaux, S.; Tourneau, T.; Fabre, O.; Susen, S.; Van Belle, E.; et al. In vivo autologous recellularization of a tissue-engineered heart valve: Are bone marrow mesenchymal stem cells the best candidates? J. Thorac. Cardiovasc. Surg. 2007, 134, 424–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zheng, L.; Xu, X.; Song, L.; Li, Y.; Li, W.; Zhang, S.; Zhang, F.; Jin, H. Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res. Ther. 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Borazjani, A.; Tahai, M.; de Jongh Curry, A.L.; Simionescu, D.T.; Guan, J.; To, F.; Elder, S.; Liao, J. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J. Biomed. Mater. Res. Part A 2010, 94, 1100–1110. [Google Scholar] [CrossRef] [Green Version]
- Hellström, M.; Moreno-Moya, J.M.; Bandstein, S.; Bom, E.; Akouri, R.R.; Miyazaki, K.; Maruyama, T.; Brännström, M. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 2016, 106, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfi, A.; Bianchi, G.; Notaro, R.; Luzzatto, L.; Cancedda, R.; Quarto, R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002, 8, 901–910. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Ren, Z.; Zhao, B.; Zhang, L.; Chen, J.; Xu, W.; Lu, S.; Zhao, Q.; Peng, J. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci. Lett. 2012, 514, 96–101. [Google Scholar] [CrossRef]
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernandez-Aviles, F.; Campistol, J.; Samitier, J.; Montserrat, N. Tissue engineering by decellularization and 3D bioprinting. Mater. Today 2017, 20, 166–178. [Google Scholar] [CrossRef] [Green Version]
- VeDepo, M.; Buse, E.; Paul, A.; Hopkins, R.; Converse, G. Comparison of Candidate Cell Populations for the Recellularization of Decellularized Heart Valves. Cell. Mol. Bioeng. 2018, 11, 197–209. [Google Scholar] [CrossRef]
- Arakelian, L.; Caille, C.; Faivre, L.; Corté, L.; Bruneval, P.; Shamdani, S.; Flageollet, C.; Albanese, P.; Domet, T.; Jarraya, M.; et al. A clinical-grade acellular matrix for esophageal replacement. J. Tissue Eng. Regen. Med. 2019, 13, 2191–2203. [Google Scholar] [CrossRef]
- Wallis, J.M.; Borg, Z.D.; Daly, A.B.; Deng, B.; Ballif, B.A.; Allen, G.B.; Jaworski, D.M.; Weiss, D.J. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng. Part C Methods 2012, 18, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Crabbé, A.; Liu, Y.; Sarker, S.F.; Bonenfant, N.R.; Barrila, J.; Borg, Z.D.; Lee, J.J.; Weiss, D.J.; Nickerson, C.A. Recellularization of decellularized lung scaffolds is enhanced by dynamic suspension culture. PLoS ONE 2015, 10, e0126846. [Google Scholar] [CrossRef]
- Sokocevic, D.; Bonenfant, N.R.; Wagner, D.E.; Borg, Z.D.; Lathrop, M.J.; Lam, Y.W.; Deng, B.; Michael, J.; DeSarno, M.J.; Ashikaga, T.; et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 2013, 34, 3256–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papalamprou, A.; Chang, C.W.; Vapniarsky, N.; Clark, A.; Walker, N.; Griffiths, L.G. Xenogeneic cardiac extracellular matrix scaffolds with or without seeded mesenchymal stem cells exhibit distinct in vivo immunosuppressive and regenerative properties. Acta Biomater. 2016, 45, 155–168. [Google Scholar] [CrossRef]
- Tiemann, T.; Padma, A.; Sehic, E.; Bäckdahl, H.; Oltean, M.; Song, M.; Brännström, M.; Hellström, M. Towards uterus tissue engineering: A comparative study of sheep uterus decellularisation. Mol. Hum. Reprod. 2020, 26, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinescu, A.; Andrei, E.; Iordache, F.; Constantinescu, E.; Maniu, H. Recellularization potential assessment of Wharton’s Jelly-derived endothelial progenitor cells using a human fetal vascular tissue model. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Coutu, D.L.; Mahfouz, W.; Loutochin, O.; Galipeau, J.; Corcos, J. Tissue engineering of rat bladder using marrow-derived mesenchymal stem cells and bladder acellular matrix. PLoS ONE 2014, 9, e111966. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, W.; Geng, L.; Zhang, L.; Liu, R.; Chen, W.; Yao, G.; Zhang, H.; Feng, X.; Gao, X.; et al. Mesenchymal stem cells from patients with rheumatoid arthritis display impaired function in inhibiting Th17 cells. J. Immunol. Res. 2015, 2015, 13. [Google Scholar] [CrossRef]
- Minehara, H.; Urabe, K.; Naruse, K.; Mehlhorn, A.T.; Uchida, K.; Südkamp, N.P.; Itoman, M. A new technique for seeding chondrocytes onto solvent-preserved human meniscus using the chemokinetic effect of recombinant human bone morphogenetic protein-2. Cell Tissue Bank. 2011, 12, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Nordberg, R.C.; Charoenpanich, A.; Vaughn, C.E.; Griffith, E.H.; Fisher, M.B.; Cole, J.H.; Spang, J.T.; Loboa, E.G. Enhanced cellular infiltration of human adipose-derived stem cells in allograft menisci using a needle-punch method. J. Orthop. Surg. Res. 2016, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, D.E.; Bonenfant, N.R.; Parsons, C.S.; Sokocevic, D.; Brooks, E.M.; Borg, Z.D.; Lathrop, M.J.; Wallis, J.D.; Daly, A.B.; Lam, Y.W.; et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 2014, 35, 3281–3297. [Google Scholar] [CrossRef] [Green Version]
- Katsimpoulas, M.; Morticelli, L.; Gontika, I.; Kouvaka, A.; Mallis, P.; Dipresa, D.; Böer, U.; Soudah, B.; Haverich, A.; Michalopoulos, E.; et al. Biocompatibility and immunogenicity of decellularized allogeneic aorta in the orthotopic rat model. Tissue Eng. Part A 2019, 25, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Hundepool, C.A.; Nijhuis, T.H.; Kotsougiani, D.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Optimizing decellularization techniques to create a new nerve allograft: An in vitro study using rodent nerve segments. Neurosurg. Focus 2017, 42, E4. [Google Scholar] [CrossRef]
- Tchoukalova, Y.D.; Hintze, J.M.; Hayden, R.E.; Lott, D.G. Tracheal decellularization using a combination of chemical, physical and bioreactor methods. Int. J. Artif. Organs 2018, 41, 100–107. [Google Scholar] [CrossRef]
- Lopera, H.M.; Griffiths, L.G. Antigen removal process preserves function of small diameter venous valved conduits, whereas SDS-decellularization results in significant valvular insufficiency. Acta Biomater. 2020, 107, 115–128. [Google Scholar] [CrossRef]
- Wong, M.L.; Griffiths, L.G. Immunogenicity in xenogeneic scaffold generation: Antigen removal vs. decellularization. Acta Biomater. 2014, 10, 1806–1816. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.L.; Leach, J.K.; Athanasiou, K.A.; Griffiths, L.G. The role of protein solubilization in antigen removal from xenogeneic tissue for heart valve tissue engineering. Biomaterials 2011, 32, 8129–8138. [Google Scholar] [CrossRef] [PubMed]
- Bielli, A.; Bernardini, R.; Varvaras, D.; Rossi, P.; Di Blasi, G.; Petrella, G.; Buonomo, O.C.; Mattei, M.; Orlandi, A. Characterization of a new decellularized bovine pericardial biological mesh: Structural and mechanical properties. J. Mech. Behav. Biomed. Mater. 2018, 78, 420–426. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [Green Version]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef] [PubMed]
- Bonenfant, N.R.; Sokocevic, D.; Wagner, D.E.; Borg, Z.D.; Lathrop, M.J.; Lam, Y.W.; Deng, B.; DeSarno, M.J.; Ashikaga, T.; Loi, R.; et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials 2013, 34, 3231–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, S.C.; Shoichet, M.S. Design of three-dimensional biomimetic scaffolds. J. Biomed. Mater. Res. Part A 2010, 94, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, Y.; Huang, Y.; Xiong, Y.; Xu, Y.; Li, X.; Lu, J.; Wang, L.; Wang, Y.; Lu, Y.; et al. Constructing heparin-modified pancreatic decellularized scaffold to improve its re-endothelialization. J. Biomater. Appl. 2018, 32, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Assmann, A.; Delfs, C.; Munakata, H.; Schiffer, F.; Horstkötter, K.; Huynh, K.; Barth, M.; Stoldt, V.R.; Kamiya, H.; Boeken, U.; et al. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials 2013, 34, 6015–6026. [Google Scholar] [CrossRef] [PubMed]
- Tang-Quan, K.R.; Xi, Y.; Hochman-Mendez, C.; Xiang, Q.; Lee, P.-F.; Sampaio, L.C.; Taylor, D.A. Gelatin Promotes Cell Retention Within Decellularized Heart Extracellular Matrix Vasculature and Parenchyma. Cell. Mol. Bioeng. 2020, 13, 633–645. [Google Scholar] [CrossRef]
- Saleh, T.; Ahmed, E.; Yu, L.; Song, S.H.; Park, K.M.; Kwak, H.H.; Woo, H.-M. Conjugating Homogenized Liver-Extracellular Matrix into Decellularized Hepatic Scaffold for Liver Tissue Engineering. J. Biomed. Mater. Res. Part A 2020, 108, 1991–2004. [Google Scholar] [CrossRef]
- Dai, J.; Qiao, W.; Shi, J.; Liu, C.; Hu, X.; Dong, N. Modifying decellularized aortic valve scaffolds with stromal cell-derived factor-1α loaded proteolytically degradable hydrogel for recellularization and remodeling. Acta Biomater. 2019, 88, 280–292. [Google Scholar] [CrossRef]
- Dong, X.; Wei, X.; Yi, W.; Gu, C.; Kang, X.; Liu, Y.; Li, Q.; Yi, D. RGD-modified acellular bovine pericardium as a bioprosthetic scaffold for tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 2327–2336. [Google Scholar] [CrossRef]
- Li, C.-Y.; Wu, X.-Y.; Tong, J.-B.; Yang, X.-X.; Zhao, J.-L.; Zheng, Q.-F.; Zhao, G.-B.; Ma, Z.-J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kitahara, H.; Yagi, H.; Tajima, K.; Okamoto, K.; Yoshitake, A.; Aeba, R.; Kudo, M.; Kashima, I.; Kawaguchi, S.; Hirano, A.; et al. Heterotopic transplantation of a decellularized and recellularized whole porcine heart. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in cardiac tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 169–187. [Google Scholar] [CrossRef] [Green Version]
- Kojima, H.; Yasuchika, K.; Fukumitsu, K.; Ishii, T.; Ogiso, S.; Miyauchi, Y.; Yamaoka, R.; Kawai, T.; Katayama, H.; Yoshitoshi-Uebayashi, E.Y.; et al. Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells. Am. J. Transplant. 2018, 18, 1351–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementi, A.; Egger, D.; Charwat, V.; Kasper, C. Cell culture conditions: Cultivation of stem cells under dynamic conditions. Cell Eng. Regen. 2020, 415–447. [Google Scholar]
- Syedain, Z.H.; Bradee, A.R.; Kren, S.; Taylor, D.A.; Tranquillo, R.T. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng. Part A 2013, 19, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Weidenhamer, N.K.; Moore, D.L.; Lobo, F.L.; Klair, N.T.; Tranquillo, R.T. Influence of culture conditions and extracellular matrix alignment on human mesenchymal stem cells invasion into decellularized engineered tissues. J. Tissue Eng. Regen. Med. 2015, 9, 605–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering human myocardium on native extracellular matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasui, H.; Lee, J.-K.; Yoshida, A.; Yokoyama, T.; Nakanishi, H.; Miwa, K.; Naito, A.T.; Oka, T.; Akazawa, H.; Nakai, J.; et al. Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials 2014, 35, 7839–7850. [Google Scholar] [CrossRef]
- Fathi, I.; Eltawila, A. Whole-liver decellularization: Advances and insights into current understanding. Xenotransplant. New Insights 2017, 139. [Google Scholar]
- Isakova, I.A.; Lanclos, C.; Bruhn, J.; Kuroda, M.J.; Baker, K.C.; Krishnappa, V.; Phinney, D.G. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS ONE 2014, 9, e87238. [Google Scholar] [CrossRef]
- Cho, P.S.; Messina, D.J.; Hirsh, E.L.; Chi, N.; Goldman, S.N.; Lo, D.P.; Harris, I.R.; Popma, S.H.; Sachs, D.H.; Huang, C.A. Immunogenicity of umbilical cord tissue–derived cells. Blood J. Am. Soc. Hematol. 2008, 111, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [Green Version]
- Baptista, L.S.; Silva, K.R.; Borojevic, R. Obesity and weight loss could alter the properties of adipose stem cells? World J. Stem Cells 2015, 7, 165. [Google Scholar] [CrossRef]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue–derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Lau, C.; Lie, A.; Chan, G.; Mok, M. Defective phenotype of mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus 2010, 19, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lei, H.; Dong, P.; Fu, X.; Yang, Z.; Yang, Y.; Ma, J.; Liu, X.; Cao, Y.; Xiao, R. Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell Transplant. 2017, 26, 1505–1519. [Google Scholar] [CrossRef] [Green Version]
- Block, T.J.; Marinkovic, M.; Tran, O.N.; Gonzalez, A.O.; Marshall, A.; Dean, D.D.; Chen, X.-D. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mo, M.; Wang, S.; Zhou, Y.; Li, H.; Wu, Y. Mesenchymal stem cell subpopulations: Phenotype, property and therapeutic potential. Cell Mol. Life Sci. 2016, 73, 3311–3321. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019, 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- McLeod, C.; Mauck, R. On the origin and impact of mesenchymal stem cell heterogeneity: New insights and emerging tools for single cell analysis. Eur. Cell Mater. 2017, 34, 217–231. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Ramasamy, R.; Lam, E.W.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia 2007, 21, 304–310. [Google Scholar] [CrossRef] [PubMed]



| Scaffold Source [Ref.] | Decellularization | Recellularization | Cells (Number) | In Vivo | Main Outputs |
|---|---|---|---|---|---|
| Aortic valve-Ovine [83] | Thawing, osmotic shock, TX-100, sodium lauroyl sarcosine, and benzonase | Seeded into valve lumen in the static chamber incubated for 2 wk. | BM-MNCs, BM-MSCs, VIC | - | A high concentration of BM-MSCs showed a good phenotype and improved the mechanical and biochemical characteristics of scaffolds. |
| Esophagus-Pig [84] | 2% SDS, 5 mM EDTA, hypotonic water, DNase | Incubated inside a rotating agitator for 21 d. | BM-MSC (2.5 × 105) | - | Successfully attach and proliferate throughout the acellular esophageal wall. |
| Lung-Rhesus macaque [30] | TX-100, SDC, NaCl, DNase | Inoculation of cells into the lungs, then and slicing and culturing for 7 d. | Rhesus primary BM-MSC and AD-MSC | - | MSCs obtained from either BM-MSCs or AD-MSCs are suitable to recellularized the lung scaffolds. |
| Lung-Rat [12] | TX-100, SDS | Injecting of cells through the pulmonary artery and pulmonary vein. | Lung ECs (4 × 107); AD-MSCs (1 × 107) | lung was replaced for 3 h | Differentiation into perivascular cells; Upregulated angiogenic growth factors; Increase ECs proliferation and survival. |
| Lung-Mouse [85] | Triton/SDC-based SDS-based CHAPS-based | Intratracheal injection. | BM-MSCs; C10 epithelial cells culturing for 1 or 14 days (2 × 106) | - | BM-MSCs localized the regions enriched with FN, Col I, IV, and laminin. No differences in attachment and proliferation among various scaffolds. |
| Lung-Mouse [86] | TX-100; SDS | Intratracheal injection; then connected to RWV for 10, 24 d. | BM-MSCs EC cell line (4 × 106) | - | BM-MSC growth and differentiation into fibroblast-like cells. |
| Lung-Mouse [87] | 1% TX-100, 2% SDC, 1 M NaCl, DNase | Inoculated to the lung for 30 min, sliced lung, and incubated for 28 d. | MSCs or lung ECs (1 × 106) | - | Binding, proliferation, and viability were not good in the densely fibrotic lung or the emphysematous lung. |
| Lung-Mouse [27] | 0.1% TX-100, 2% SDC, DNase. | Intratracheal inoculation. cultured for 1 month in different media. | BM-MSCs (2 × 106) | - | Attached well in regions enriched with collagen I and IV and laminin. Proliferation well in MSCs basal medium. |
| Myocardial patches-Rat [88] | AR using ASB-14 | Placing of cell and culturing for 7 days. | Human or murine MSCs (1000 cell/mm2) | implanted in adult male mice for 12 wk | Inflammatory responses were altered Unexpectedly, AR- MSC intense inflammatory reaction. |
| Ovary-Mouse [89] | P1: 0.5% SDS P2: 2% SDC P3: P1 + P2. | 5 successive injections culturing for 5 days. | BM-MSCs (2 × 105) | - | Cells distributed within scaffolds Good recellularization and proliferation capacity. |
| Pancreas-Mouse [13] | 1% T-100, 0.1% ammonia, DNase 200 U/mL. | Inoculated through the portal vein and cultured for 5 d. | hPL-MSC (6−10 × 105) | implanted to pancreas for 45 days | MSCs differentiation; bioengineered functional pancreas generated. |
| Pulm. valve conduits-Pig [74] | Tris, EDTA, aprotinin, SDS, Tris NaCl buffer | Injection into the pulmonary arterial wall and the annulus of the pulmonary valve. | autologous BM-MNCs or BM-MSCs | Native pulmonary valves were replaced. | Increasing recolonization; altering the inflammation and structural deterioration; paracrine factors secreted stimulate cells differentiation. |
| Sciatic nerve- Rat [81] | 3% TX-100, 4% SDC | Cells were used to recellularize acellular nerve. | Schwann like-cells (from BM-MSCs, and AD-MSCs) (5 × 105) | Grafting of the left sciatic nerve | Promoting nerve regeneration; Protection against muscle atrophy; Increase sensitivity to stimulus. |
| Trachea–Pig [59] | P1: TX-100 P2: hypotonic sol + hypertonic sol | Direct transplantation + inoculated on external and luminal surfaces. | MSC TEC | samples collected after 7 and 21 days P. O. | Altering mononuclear cellular infiltration. |
| Umbilical artery-Human [90] | CHAPS buffer, SDS | Cells were incubated for 96 h. | EPCs from Wharton jelly MSCs | Regenerating injured vascular tissue. | |
| Urinary Bladder–Rat [91] | 1% SDS; TX-100; DNase | seeded on both sides of the bladder segment and culturing for 7 d. | BM-MSCs (1 × 106) | Implantation following Partial cystectomy. | Immunomodulatory action Enhanced muscle regeneration and tissue remodeling. |
| Uterus-Rat [79] | 1%TX-100 + 4%DMSO + PBS; 1%TX-100 + 4%DMSO + H2O; 2%SDC + H2O | Multiple direct injections | Primary uterine cells + BM-MSCs culturing for 3 d (7.3 × 106) | Grafting of intrauterine defects | Modulating the immune response Differentiation into uterine specific cells. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, E.; Saleh, T.; Xu, M. Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells. Cells 2021, 10, 1787. https://doi.org/10.3390/cells10071787
Ahmed E, Saleh T, Xu M. Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells. Cells. 2021; 10(7):1787. https://doi.org/10.3390/cells10071787
Chicago/Turabian StyleAhmed, Ebtehal, Tarek Saleh, and Meifeng Xu. 2021. "Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells" Cells 10, no. 7: 1787. https://doi.org/10.3390/cells10071787