Neuronal Circuit Dysfunction in Amyotrophic Lateral Sclerosis
Abstract
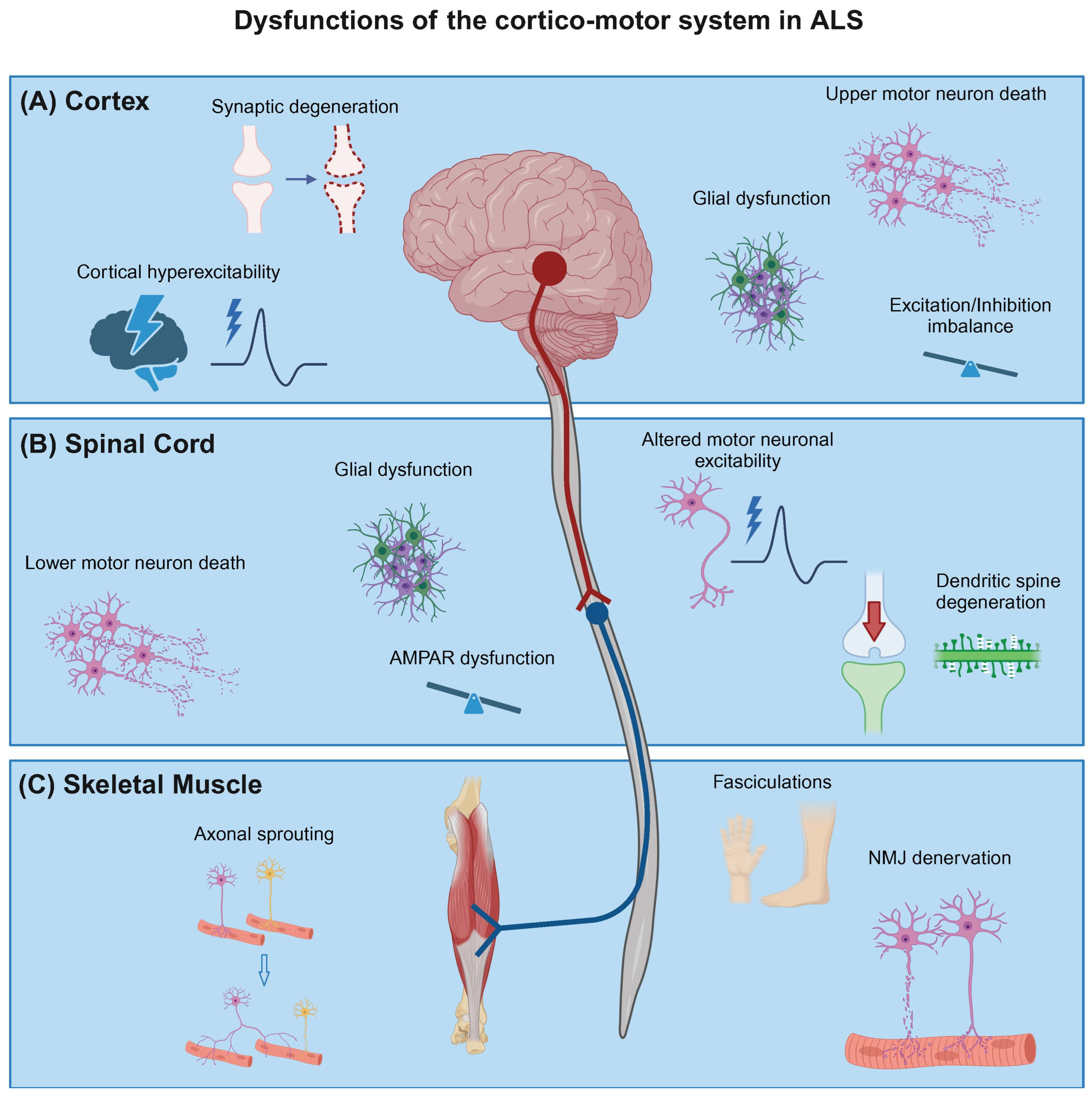
:1. Introduction
2. Cortical Neuron Dysfunction in ALS
3. Altered Excitability in the Lower Motor Neurons in ALS
4. Glutamate Receptor Dysregulation in ALS
5. Neuromuscular Junction Degeneration in ALS
6. Glial Contribution towards Neuronal Circuit Dysfunction in ALS
7. Use of Human Stem Cell Models to Study Neuronal Circuit Dysfunction in ALS
Author Contributions
Funding
Conflicts of Interest
References
- Lemon, R.N. Descending Pathways in Motor Control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Yoshida, Y.; Isa, T. Neural and Genetic Basis of Dexterous Hand Movements. Curr. Opin. Neurobiol. 2018, 52, 25–32. [Google Scholar] [CrossRef]
- Eisen, A. The Dying Forward Hypothesis of ALS: Tracing Its History. Brain Sci. 2021, 11, 300. [Google Scholar] [CrossRef]
- Braak, H.; Brettschneider, J.; Ludolph, A.C.; Lee, V.M.; Trojanowski, J.Q.; Tredici, K.D. Amyotrophic Lateral Sclerosis—A Model of Corticofugal Axonal Spread. Nat. Rev. Neurol. 2013, 9, 708–714. [Google Scholar] [CrossRef]
- Baker, M.R. ALS—Dying Forward, Backward or Outward? Nat. Rev. Neurol. 2014, 10, 660. [Google Scholar] [CrossRef]
- Verma, S.; Khurana, S.; Vats, A.; Sahu, B.; Ganguly, N.K.; Chakraborti, P.; Gourie-Devi, M.; Taneja, V. Neuromuscular Junction Dysfunction in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1502–1527. [Google Scholar] [CrossRef]
- Fischer, L.R.; Culver, D.G.; Tennant, P.; Davis, A.A.; Wang, M.; Castellano-Sanchez, A.; Khan, J.; Polak, M.A.; Glass, J.D. Amyotrophic Lateral Sclerosis Is a Distal Axonopathy: Evidence in Mice and Man. Exp. Neurol. 2004, 185, 232–240. [Google Scholar] [CrossRef]
- Dadon-Nachum, M.; Melamed, E.; Offen, D. The “Dying-Back” Phenomenon of Motor Neurons in ALS. J. Mol. Neurosci. 2011, 43, 470–477. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef]
- Ryan, M.; Heverin, M.; McLaughlin, R.L.; Hardiman, O. Lifetime Risk and Heritability of Amyotrophic Lateral Sclerosis. JAMA Neurol. 2019, 76, 1367. [Google Scholar] [CrossRef]
- Wingo, T.S.; Cutler, D.J.; Yarab, N.; Kelly, C.M.; Glass, J.D. The Heritability of Amyotrophic Lateral Sclerosis in a Clinically Ascertained United States Research Registry. PLoS ONE 2011, 6, e27985. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.D.; Bonini, N.M. Repeat-Associated Non-AUG (RAN) Translation Mechanisms Are Running into Focus for GGGGCC-Repeat Associated ALS/FTD. Prog. Neurobiol. 2019, 183, 101697. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.-X.; et al. Mutations in Cu/Zn Superoxide Dismutase Gene Are Associated with Familial Amyotrophic Lateral Sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 Mutations Associated with Amyotrophic Lateral Sclerosis Analysis of Variant Severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef]
- Rothstein, J.D. Current Hypotheses for the Underlying Biology of Amyotrophic Lateral Sclerosis. Ann. Neurol. 2009, 65 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- Saccon, R.A.; Bunton-Stasyshyn, R.K.A.; Fisher, E.M.C.; Fratta, P. Is SOD1 Loss of Function Involved in Amyotrophic Lateral Sclerosis? Brain 2013, 136, 2342–2358. [Google Scholar] [CrossRef]
- Shatunov, A.; Al-Chalabi, A. The Genetic Architecture of ALS. Neurobiol. Dis. 2021, 147, 105156. [Google Scholar] [CrossRef]
- Klim, J.R.; Williams, L.A.; Limone, F.; Guerra San Juan, I.; Davis-Dusenbery, B.N.; Mordes, D.A.; Burberry, A.; Steinbaugh, M.J.; Gamage, K.K.; Kirchner, R.; et al. ALS-Implicated Protein TDP-43 Sustains Levels of STMN2, a Mediator of Motor Neuron Growth and Repair. Nat. Neurosci. 2019, 22, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Melamed, Z.; López-Erauskin, J.; Baughn, M.W.; Zhang, O.; Drenner, K.; Sun, Y.; Freyermuth, F.; McMahon, M.A.; Beccari, M.S.; Artates, J.W.; et al. Premature Polyadenylation-Mediated Loss of Stathmin-2 Is a Hallmark of TDP-43-Dependent Neurodegeneration. Nat. Neurosci. 2019, 22, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.-L.; Wilkins, O.G.; Keuss, M.J.; Hill, S.E.; Zanovello, M.; Lee, W.C.; Bampton, A.; Lee, F.C.Y.; Masino, L.; Qi, Y.A.; et al. TDP-43 Loss and ALS-Risk SNPs Drive Mis-Splicing and Depletion of UNC13A. Nature 2022, 603, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Suk, T.R.; Rousseaux, M.W.C. The Role of TDP-43 Mislocalization in Amyotrophic Lateral Sclerosis. Mol. Neurodegener. 2020, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic Lateral Sclerosis: A Neurodegenerative Disorder Poised for Successful Therapeutic Translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Gunes, Z.I.; Kan, V.W.Y.; Ye, X.; Liebscher, S. Exciting Complexity: The Role of Motor Circuit Elements in ALS Pathophysiology. Front. Neurosci. 2020, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Espinosa, P.M.; Powell, K.L.; O’Brien, T.J. Regulators of Synaptic Transmission: Roles in the Pathogenesis and Treatment of Epilepsy. Epilepsia 2012, 53, 41–58. [Google Scholar] [CrossRef]
- Styr, B.; Slutsky, I. Imbalance between Firing Homeostasis and Synaptic Plasticity Drives Early-Phase Alzheimer’s Disease. Nat. Neurosci. 2018, 21, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Sattler, R. Synaptic Dysfunction and Altered Excitability in C9ORF72 ALS/FTD. Brain Res. 2018, 1693, 98–108. [Google Scholar] [CrossRef]
- Mora, S.; Allodi, I. Neural Circuit and Synaptic Dysfunctions in ALS-FTD Pathology. Front. Neural Circuits 2023, 17, 1208876. [Google Scholar] [CrossRef]
- Ling, S.-C. Synaptic Paths to Neurodegeneration: The Emerging Role of TDP-43 and FUS in Synaptic Functions. Neural Plast. 2018, 2018, 8413496. [Google Scholar] [CrossRef]
- Kassa, R.M.; Bonafede, R.; Boschi, F.; Malatesta, M.; Mariotti, R. The Role of Mutated SOD1 Gene in Synaptic Stripping and MHC Class I Expression Following Nerve Axotomy in ALS Murine Model. Eur. J. Histochem. 2018, 62, 2904. [Google Scholar] [CrossRef]
- Xiao, S.; McKeever, P.M.; Lau, A.; Robertson, J. Synaptic Localization of C9orf72 Regulates Post-Synaptic Glutamate Receptor 1 Levels. Acta Neuropathol. Commun. 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Rothstein, J.D. Rodent Models of Amyotrophic Lateral Sclerosis. Curr. Protoc. Pharmacol. 2015, 69, 5.67.1–5.67.21. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Robberecht, W.; Van Den Bosch, L. Modelling Amyotrophic Lateral Sclerosis: Progress and Possibilities. Dis. Model. Mech. 2017, 10, 537–549. [Google Scholar] [CrossRef]
- Lamas, N.J.; Roybon, L. Harnessing the Potential of Human Pluripotent Stem Cell-Derived Motor Neurons for Drug Discovery in Amyotrophic Lateral Sclerosis: From the Clinic to the Laboratory and Back to the Patient. Front. Drug Discov. 2021, 1, 773424. [Google Scholar] [CrossRef]
- Aggarwal, S.; Cudkowicz, M. ALS Drug Development: Reflections from the Past and a Way Forward. Neurotherapeutics 2008, 5, 516–527. [Google Scholar] [CrossRef]
- VINCENT, A. Strategic Approaches to Developing Drug Treatments for ALS. Drug Discov. Today 2008, 13, 67–72. [Google Scholar] [CrossRef]
- Lemon, R.N.; Griffiths, J. Comparing the Function of the Corticospinal System in Different Species: Organizational Differences for Motor Specialization? Muscle Nerve 2005, 32, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D Human Induced Pluripotent Stem Cell-Derived Cultures for Neurodegenerative Disease Modelling. Mol. Neurodegener. 2018, 13, 27. [Google Scholar] [CrossRef]
- Giacomelli, E.; Vahsen, B.F.; Calder, E.L.; Xu, Y.; Scaber, J.; Gray, E.; Dafinca, R.; Talbot, K.; Studer, L. Human Stem Cell Models of Neurodegeneration: From Basic Science of Amyotrophic Lateral Sclerosis to Clinical Translation. Cell Stem Cell 2022, 29, 11–35. [Google Scholar] [CrossRef]
- Faravelli, I.; Costamagna, G.; Tamanini, S.; Corti, S. Back to the Origins: Human Brain Organoids to Investigate Neurodegeneration. Brain Res. 2020, 1727, 146561. [Google Scholar] [CrossRef]
- Menon, P.; Vucic, S. The Upper Motor Neuron—Improved Knowledge from ALS and Related Clinical Disorders. Brain Sci. 2021, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Roccatagliata, L.; Bonzano, L.; Mancardi, G.; Canepa, C.; Caponnetto, C. Detection of Motor Cortex Thinning and Corticospinal Tract Involvement by Quantitative MRI in Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2009, 10, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mezzapesa, D.M.; D’Errico, E.; Tortelli, R.; Distaso, E.; Cortese, R.; Tursi, M.; Federico, F.; Zoccolella, S.; Logroscino, G.; Dicuonzo, F.; et al. Cortical Thinning and Clinical Heterogeneity in Amyotrophic Lateral Sclerosis. PLoS ONE 2013, 8, e80748. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, E.; Veldink, J.H.; Hendrikse, J.; Schelhaas, H.J.; van den Heuvel, M.P.; van den Berg, L.H. Structural MRI Reveals Cortical Thinning in Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Genç, B.; Jara, J.H.; Lagrimas, A.K.B.; Pytel, P.; Roos, R.P.; Mesulam, M.M.; Geula, C.; Bigio, E.H.; Özdinler, P.H. Apical Dendrite Degeneration, a Novel Cellular Pathology for Betz Cells in ALS. Sci. Rep. 2017, 7, 41765. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.J. Driven to Decay: Excitability and Synaptic Abnormalities in Amyotrophic Lateral Sclerosis. Brain Res. Bull. 2018, 140, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, C.M.; Sideris, D.I.; Carroll, E.; Rotariu, S.; Salomonsson, S.; Tzioras, M.; McKenzie, C.-A.; Smith, C.; von Arnim, C.A.F.; Ludolph, A.C.; et al. Synapse Loss in the Prefrontal Cortex Is Associated with Cognitive Decline in Amyotrophic Lateral Sclerosis. Acta Neuropathol. 2018, 135, 213–226. [Google Scholar] [CrossRef]
- Dadar, M.; Manera, A.L.; Zinman, L.; Korngut, L.; Genge, A.; Graham, S.J.; Frayne, R.; Collins, D.L.; Kalra, S. Cerebral Atrophy in Amyotrophic Lateral Sclerosis Parallels the Pathological Distribution of TDP43. Brain Commun. 2020, 2, fcaa061. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Burg, T.; Scekic-Zahirovic, J.; Fischer, M.; Rouaux, C. Upper and Lower Motor Neuron Degenerations Are Somatotopically Related and Temporally Ordered in the Sod1 Mouse Model of Amyotrophic Lateral Sclerosis. Brain Sci. 2021, 11, 369. [Google Scholar] [CrossRef]
- Özdinler, P.H.; Benn, S.; Yamamoto, T.H.; Güzel, M.; Brown, R.H.; Macklis, J.D. Corticospinal Motor Neurons and Related Subcerebral Projection Neurons Undergo Early and Specific Neurodegeneration in HSOD1 G93A Transgenic ALS Mice. J. Neurosci. 2011, 31, 4166–4177. [Google Scholar] [CrossRef]
- Walker, A.K.; Spiller, K.J.; Ge, G.; Zheng, A.; Xu, Y.; Zhou, M.; Tripathy, K.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M.-Y. Functional Recovery in New Mouse Models of ALS/FTLD after Clearance of Pathological Cytoplasmic TDP-43. Acta Neuropathol. 2015, 130, 643–660. [Google Scholar] [CrossRef]
- Wegorzewska, I.; Bell, S.; Cairns, N.J.; Miller, T.M.; Baloh, R.H. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 18809–18814. [Google Scholar] [CrossRef]
- Vucic, S.; Nicholson, G.A.; Kiernan, M.C. Cortical Hyperexcitability May Precede the Onset of Familial Amyotrophic Lateral Sclerosis. Brain 2008, 131, 1540–1550. [Google Scholar] [CrossRef]
- Grieve, S.M.; Menon, P.; Korgaonkar, M.S.; Gomes, L.; Foster, S.; Kiernan, M.C.; Vucic, S. Potential Structural and Functional Biomarkers of Upper Motor Neuron Dysfunction in ALS. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 85–92. [Google Scholar] [CrossRef]
- Geevasinga, N.; Van den Bos, M.; Menon, P.; Vucic, S. Utility of Transcranial Magnetic Simulation in Studying Upper Motor Neuron Dysfunction in Amyotrophic Lateral Sclerosis. Brain Sci. 2021, 11, 906. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Xie, M.; Pallegar, P.N.; Parusel, S.; Nguyen, A.T.; Wu, L.-J. Regulation of Cortical Hyperexcitability in Amyotrophic Lateral Sclerosis: Focusing on Glial Mechanisms. Mol. Neurodegener. 2023, 18, 75. [Google Scholar] [CrossRef]
- Timmins, H.C.; Vucic, S.; Kiernan, M.C. Cortical Hyperexcitability in Amyotrophic Lateral Sclerosis: From Pathogenesis to Diagnosis. Curr. Opin. Neurol. 2023, 36, 353–359. [Google Scholar] [CrossRef]
- Agarwal, S.; Highton-Williamson, E.; Caga, J.; Howells, J.; Dharmadasa, T.; Matamala, J.M.; Ma, Y.; Shibuya, K.; Hodges, J.R.; Ahmed, R.M.; et al. Motor Cortical Excitability Predicts Cognitive Phenotypes in Amyotrophic Lateral Sclerosis. Sci. Rep. 2021, 11, 2172. [Google Scholar] [CrossRef]
- Douaud, G.; Filippini, N.; Knight, S.; Talbot, K.; Turner, M.R. Integration of Structural and Functional Magnetic Resonance Imaging in Amyotrophic Lateral Sclerosis. Brain 2011, 134, 3470–3479. [Google Scholar] [CrossRef]
- Schmidt, R.; Verstraete, E.; de Reus, M.A.; Veldink, J.H.; van den Berg, L.H.; van den Heuvel, M.P. Correlation between Structural and Functional Connectivity Impairment in Amyotrophic Lateral Sclerosis. Hum. Brain Mapp. 2014, 35, 4386–4395. [Google Scholar] [CrossRef]
- Iyer, P.M.; Egan, C.; Pinto-Grau, M.; Burke, T.; Elamin, M.; Nasseroleslami, B.; Pender, N.; Lalor, E.C.; Hardiman, O. Functional Connectivity Changes in Resting-State EEG as Potential Biomarker for Amyotrophic Lateral Sclerosis. PLoS ONE 2015, 10, e0128682. [Google Scholar] [CrossRef]
- Proudfoot, M.; Rohenkohl, G.; Quinn, A.; Colclough, G.L.; Wuu, J.; Talbot, K.; Woolrich, M.W.; Benatar, M.; Nobre, A.C.; Turner, M.R. Altered Cortical Beta-band Oscillations Reflect Motor System Degeneration in Amyotrophic Lateral Sclerosis. Hum. Brain Mapp. 2017, 38, 237–254. [Google Scholar] [CrossRef]
- Proudfoot, M.; Colclough, G.L.; Quinn, A.; Wuu, J.; Talbot, K.; Benatar, M.; Nobre, A.C.; Woolrich, M.W.; Turner, M.R. Increased Cerebral Functional Connectivity in ALS. Neurology 2018, 90, e1418–e1424. [Google Scholar] [CrossRef]
- Kim, J.; Hughes, E.G.; Shetty, A.S.; Arlotta, P.; Goff, L.A.; Bergles, D.E.; Brown, S.P. Changes in the Excitability of Neocortical Neurons in a Mouse Model of Amyotrophic Lateral Sclerosis Are Not Specific to Corticospinal Neurons and Are Modulated by Advancing Disease. J. Neurosci. 2017, 37, 9037–9053. [Google Scholar] [CrossRef]
- Dyer, M.S.; Reale, L.A.; Lewis, K.E.; Walker, A.K.; Dickson, T.C.; Woodhouse, A.; Blizzard, C.A. Mislocalisation of TDP-43 to the Cytoplasm Causes Cortical Hyperexcitability and Reduced Excitatory Neurotransmission in the Motor Cortex. J. Neurochem. 2021, 157, 1300–1315. [Google Scholar] [CrossRef]
- Scekic-Zahirovic, J.; Benetton, C.; Brunet, A.; Ye, X.; Logunov, E.; Douchamps, V.; Megat, S.; Andry, V.; Kan, V.W.Y.; Stuart-Lopez, G.; et al. Cortical Hyperexcitability in Mouse Models and Patients with Amyotrophic Lateral Sclerosis Is Linked to Noradrenaline Deficiency. Sci. Transl. Med. 2024, 16, eadg3665. [Google Scholar] [CrossRef]
- Yokota, T.; Yoshino, A.; Inaba, A.; Saito, Y. Double Cortical Stimulation in Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 1996, 61, 596–600. [Google Scholar] [CrossRef]
- Nieto-Gonzalez, J.L.; Moser, J.; Lauritzen, M.; Schmitt-John, T.; Jensen, K. Reduced GABAergic Inhibition Explains Cortical Hyperexcitability in the Wobbler Mouse Model of ALS. Cereb. Cortex 2011, 21, 625–635. [Google Scholar] [CrossRef]
- Clark, R.M.; Brizuela, M.; Blizzard, C.A.; Dickson, T.C. Reduced Excitability and Increased Neurite Complexity of Cortical Interneurons in a Familial Mouse Model of Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2018, 12, 328. [Google Scholar] [CrossRef]
- Khademullah, C.S.; Aqrabawi, A.J.; Place, K.M.; Dargaei, Z.; Liang, X.; Pressey, J.C.; Bedard, S.; Yang, J.W.; Garand, D.; Keramidis, I.; et al. Cortical Interneuron-Mediated Inhibition Delays the Onset of Amyotrophic Lateral Sclerosis. Brain 2020, 143, 800–810. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Noakes, P.G.; Bellingham, M.C. Motor Cortex Layer V Pyramidal Neurons Exhibit Dendritic Regression, Spine Loss, and Increased Synaptic Excitation in the Presymptomatic HSOD1 G93A Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurosci. 2015, 35, 643–647. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Klenowski, P.M.; Lee, J.D.; Drieberg-Thompson, J.R.; Bartlett, S.E.; Ngo, S.T.; Hilliard, M.A.; Bellingham, M.C.; Noakes, P.G. Cortical Synaptic and Dendritic Spine Abnormalities in a Presymptomatic TDP-43 Model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2016, 6, 37968. [Google Scholar] [CrossRef]
- Handley, E.E.; Pitman, K.A.; Dawkins, E.; Young, K.M.; Clark, R.M.; Jiang, T.C.; Turner, B.J.; Dickson, T.C.; Blizzard, C.A. Synapse Dysfunction of Layer V Pyramidal Neurons Precedes Neurodegeneration in a Mouse Model of TDP-43 Proteinopathies. Cereb. Cortex 2016, 27, 3630–3647. [Google Scholar] [CrossRef]
- Reale, L.A.; Dyer, M.S.; Perry, S.E.; Young, K.M.; Dickson, T.C.; Woodhouse, A.; Blizzard, C.A. Pathologically Mislocalised TDP-43 in Upper Motor Neurons Causes a Die-Forward Spread of ALS-like Pathogenic Changes throughout the Mouse Corticomotor System. Prog. Neurobiol. 2023, 226, 102449. [Google Scholar] [CrossRef]
- Sloan, S.A.; Darmanis, S.; Huber, N.; Khan, T.A.; Birey, F.; Caneda, C.; Reimer, R.; Quake, S.R.; Barres, B.A.; Paşca, S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 2017, 95, 779–790.e6. [Google Scholar] [CrossRef]
- Paşca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.-Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional Cortical Neurons and Astrocytes from Human Pluripotent Stem Cells in 3D Culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Perkins, E.M.; Burr, K.; Banerjee, P.; Mehta, A.R.; Dando, O.; Selvaraj, B.T.; Suminaite, D.; Nanda, J.; Henstridge, C.M.; Gillingwater, T.H.; et al. Altered Network Properties in C9ORF72 Repeat Expansion Cortical Neurons Are Due to Synaptic Dysfunction. Mol. Neurodegener. 2021, 16, 13. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Gao, R.; Negraes, P.D.; Gu, J.; Buchanan, J.; Preissl, S.; Wang, A.; Wu, W.; Haddad, G.G.; Chaim, I.A.; et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 2019, 25, 558–569.e7. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Naderi-Meshkin, H.; Cornelius, V.A.; Eleftheriadou, M.; Potel, K.N.; Setyaningsih, W.A.W.; Margariti, A. Vascular Organoids: Unveiling Advantages, Applications, Challenges, and Disease Modelling Strategies. Stem Cell Res. Ther. 2023, 14, 292. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, J.; Xu, Z.; Yan, H.; Tang, B.; Liu, C.; Chen, C.; Meng, Q. Microglia-Containing Human Brain Organoids for the Study of Brain Development and Pathology. Mol. Psychiatry 2023, 28, 96–107. [Google Scholar] [CrossRef]
- Kofman, S.; Sun, X.; Ogbolu, V.C.; Ibric, L.; Qiang, L. Vascularized Brain Assembloids with Enhanced Cellular Complexity Provide Insights into The Cellular Deficits of Tauopathy. bioRxiv 2023. [Google Scholar] [CrossRef]
- Weskamp, K.; Tank, E.M.; Miguez, R.; McBride, J.P.; Gómez, N.B.; White, M.; Lin, Z.; Gonzalez, C.M.; Serio, A.; Sreedharan, J.; et al. Shortened TDP43 Isoforms Upregulated by Neuronal Hyperactivity Drive TDP43 Pathology in ALS. J. Clin. Investig. 2020, 130, 1139–1155. [Google Scholar] [CrossRef]
- Stifani, N. Motor Neurons and the Generation of Spinal Motor Neuron Diversity. Front. Cell Neurosci. 2014, 8, 293. [Google Scholar] [CrossRef]
- Conradi, S.; Ronnevi, L.O. Selective Vulnerability of Alpha Motor Neurons in ALS: Relation to Autoantibodies toward Acetylcholinesterase (AChE) in ALS Patients. Brain Res. Bull. 1993, 30, 369–371. [Google Scholar] [CrossRef]
- Lalancette-Hebert, M.; Sharma, A.; Lyashchenko, A.K.; Shneider, N.A. Gamma Motor Neurons Survive and Exacerbate Alpha Motor Neuron Degeneration in ALS. Proc. Natl. Acad. Sci. USA 2016, 113, E8316–E8325. [Google Scholar] [CrossRef]
- de Carvalho, M.; Swash, M. Lower Motor Neuron Dysfunction in ALS. Clin. Neurophysiol. 2016, 127, 2670–2681. [Google Scholar] [CrossRef]
- King, A.E.; Woodhouse, A.; Kirkcaldie, M.T.K.; Vickers, J.C. Excitotoxicity in ALS: Overstimulation, or Overreaction? Exp. Neurol. 2016, 275, 162–171. [Google Scholar] [CrossRef]
- Eisen, A. Amyotrophic Lateral Sclerosis: A 40-Year Personal Perspective. J. Clin. Neurosci. 2009, 16, 505–512. [Google Scholar] [CrossRef]
- Mills, K.R. Detecting Fasciculations in Amyotrophic Lateral Sclerosis: Duration of Observation Required. J. Neurol. Neurosurg. Psychiatry 2011, 82, 549–551. [Google Scholar] [CrossRef]
- Bashford, J.; Wickham, A.; Iniesta, R.; Drakakis, E.; Boutelle, M.; Mills, K.; Shaw, C. SPiQE: An Automated Analytical Tool for Detecting and Characterising Fasciculations in Amyotrophic Lateral Sclerosis. Clin. Neurophysiol. 2019, 130, 1083–1090. [Google Scholar] [CrossRef]
- Mogyoros, I.; Kiernan, M.C.; Burke, D.; Bostock, H. Ischemic Resistance of Cutaneous Afferents and Motor Axons in Patients with Amyotrophic Lateral Sclerosis. Muscle Nerve 1998, 21, 1692–1700. [Google Scholar] [CrossRef]
- Bostock, H.; Sharief, M.K.; Reid, G.; Murray, N.M.F. Axonal Ion Channel Dysfunction in Amyotrophic Lateral Sclerosis. Brain 1995, 118, 217–225. [Google Scholar] [CrossRef]
- Geevasinga, N.; Menon, P.; Özdinler, P.H.; Kiernan, M.C.; Vucic, S. Pathophysiological and Diagnostic Implications of Cortical Dysfunction in ALS. Nat. Rev. Neurol. 2016, 12, 651–661. [Google Scholar] [CrossRef]
- Bellingham, M.C. A Review of the Neural Mechanisms of Action and Clinical Efficiency of Riluzole in Treating Amyotrophic Lateral Sclerosis: What Have We Learned in the Last Decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Wainger, B.J.; Kiskinis, E.; Mellin, C.; Wiskow, O.; Han, S.S.W.; Sandoe, J.; Perez, N.P.; Williams, L.A.; Lee, S.; Boulting, G.; et al. Intrinsic Membrane Hyperexcitability of Amyotrophic Lateral Sclerosis Patient-Derived Motor Neurons. Cell Rep. 2014, 7, 1–11. [Google Scholar] [CrossRef]
- Wainger, B.J.; Macklin, E.A.; Vucic, S.; McIlduff, C.E.; Paganoni, S.; Maragakis, N.J.; Bedlack, R.; Goyal, N.A.; Rutkove, S.B.; Lange, D.J.; et al. Effect of Ezogabine on Cortical and Spinal Motor Neuron Excitability in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2021, 78, 186. [Google Scholar] [CrossRef]
- Marchand-Pauvert, V.; Peyre, I.; Lackmy-Vallee, A.; Querin, G.; Bede, P.; Lacomblez, L.; Debs, R.; Pradat, P. Absence of Hyperexcitability of Spinal Motoneurons in Patients with Amyotrophic Lateral Sclerosis. J. Physiol. 2019, 597, 5445–5467. [Google Scholar] [CrossRef]
- Martínez-Silva, M.d.L.; Imhoff-Manuel, R.D.; Sharma, A.; Heckman, C.; Shneider, N.A.; Roselli, F.; Zytnicki, D.; Manuel, M. Hypoexcitability Precedes Denervation in the Large Fast-Contracting Motor Units in Two Unrelated Mouse Models of ALS. Elife 2018, 7, e30955. [Google Scholar] [CrossRef]
- Sareen, D.; O’Rourke, J.G.; Meera, P.; Muhammad, A.K.M.G.; Grant, S.; Simpkinson, M.; Bell, S.; Carmona, S.; Ornelas, L.; Sahabian, A.; et al. Targeting RNA Foci in IPSC-Derived Motor Neurons from ALS Patients with a C9ORF72 Repeat Expansion. Sci. Transl. Med. 2013, 5, 208ra149. [Google Scholar] [CrossRef]
- Naujock, M.; Stanslowsky, N.; Bufler, S.; Naumann, M.; Reinhardt, P.; Sterneckert, J.; Kefalakes, E.; Kassebaum, C.; Bursch, F.; Lojewski, X.; et al. 4-Aminopyridine Induced Activity Rescues Hypoexcitable Motor Neurons from Amyotrophic Lateral Sclerosis Patient-Derived Induced Pluripotent Stem Cells. Stem Cells 2016, 34, 1563–1575. [Google Scholar] [CrossRef]
- Bae, J.S.; Simon, N.G.; Menon, P.; Vucic, S.; Kiernan, M.C. The Puzzling Case of Hyperexcitability in Amyotrophic Lateral Sclerosis. J. Clin. Neurol. 2013, 9, 65. [Google Scholar] [CrossRef]
- Devlin, A.-C.; Burr, K.; Borooah, S.; Foster, J.D.; Cleary, E.M.; Geti, I.; Vallier, L.; Shaw, C.E.; Chandran, S.; Miles, G.B. Human IPSC-Derived Motoneurons Harbouring TARDBP or C9ORF72 ALS Mutations Are Dysfunctional despite Maintaining Viability. Nat. Commun. 2015, 6, 5999. [Google Scholar] [CrossRef]
- Heckman, C.J.; Mottram, C.; Quinlan, K.; Theiss, R.; Schuster, J. Motoneuron Excitability: The Importance of Neuromodulatory Inputs. Clin. Neurophysiol. 2009, 120, 2040–2054. [Google Scholar] [CrossRef]
- Broadhead, M.J.; Bonthron, C.; Waddington, J.; Smith, W.V.; Lopez, M.F.; Burley, S.; Valli, J.; Zhu, F.; Komiyama, N.H.; Smith, C.; et al. Selective Vulnerability of Tripartite Synapses in Amyotrophic Lateral Sclerosis. Acta Neuropathol. 2022, 143, 471–486. [Google Scholar] [CrossRef]
- Hall, C.E.; Yao, Z.; Choi, M.; Tyzack, G.E.; Serio, A.; Luisier, R.; Harley, J.; Preza, E.; Arber, C.; Crisp, S.J.; et al. Progressive Motor Neuron Pathology and the Role of Astrocytes in a Human Stem Cell Model of VCP-Related ALS. Cell Rep. 2017, 19, 1739–1749. [Google Scholar] [CrossRef]
- Sephton, C.F.; Tang, A.A.; Kulkarni, A.; West, J.; Brooks, M.; Stubblefield, J.J.; Liu, Y.; Zhang, M.Q.; Green, C.B.; Huber, K.M.; et al. Activity-Dependent FUS Dysregulation Disrupts Synaptic Homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, E4769–E4778. [Google Scholar] [CrossRef]
- Hertz, L. The Glutamate–Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front. Endocrinol. 2013, 4, 59. [Google Scholar] [CrossRef]
- Erecińska, M.; Silver, I.A. Metabolism and Role of Glutamate in Mammalian Brain. Prog. Neurobiol. 1990, 35, 245–296. [Google Scholar] [CrossRef]
- Pradhan, J.; Bellingham, M.C. Neurophysiological Mechanisms Underlying Cortical Hyper-Excitability in Amyotrophic Lateral Sclerosis: A Review. Brain Sci. 2021, 11, 549. [Google Scholar] [CrossRef]
- Eisen, A.; Kim, S.; Pant, B. Amyotrophic Lateral Sclerosis (ALS): A Phylogenetic Disease of the Corticomotoneuron? Muscle Nerve 1992, 15, 219–224. [Google Scholar] [CrossRef]
- Vandenberghe, W.; Robberecht, W.; Brorson, J.R. AMPA Receptor Calcium Permeability, GluR2 Expression, and Selective Motoneuron Vulnerability. J. Neurosci. 2000, 20, 123–132. [Google Scholar] [CrossRef]
- Foran, E.; Trotti, D. Glutamate Transporters and the Excitotoxic Path to Motor Neuron Degeneration in Amyotrophic Lateral Sclerosis. Antioxid. Redox Signal 2009, 11, 1587–1602. [Google Scholar] [CrossRef]
- Van Den Bosch, L.; Van Damme, P.; Bogaert, E.; Robberecht, W. The Role of Excitotoxicity in the Pathogenesis of Amyotrophic Lateral Sclerosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2006, 1762, 1068–1082. [Google Scholar] [CrossRef]
- Robberecht, W.; Van Den Bosch, L.; Vleminckx, V. Amyotrophic Lateral Sclerosis: Pathogenesis. Acta Neurol. Belg. 2000, 100, 181–187. [Google Scholar] [CrossRef]
- Williams, T.L.; Day, N.C.; Ince, P.G.; Kamboj, R.K.; Shaw, P.J. Calcium-permeable A-amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid Receptors: A Molecular Determinant of Selective Vulnerability in Amyotrophic Lateral Sclerosis. Ann. Neurol. 1997, 42, 200–207. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Rothstein, J.D. From Charcot to Lou Gehrig: Deciphering Selective Motor Neuron Death in Als. Nat. Rev. Neurosci. 2001, 2, 806–819. [Google Scholar] [CrossRef]
- Selvaraj, B.T.; Livesey, M.R.; Zhao, C.; Gregory, J.M.; James, O.T.; Cleary, E.M.; Chouhan, A.K.; Gane, A.B.; Perkins, E.M.; Dando, O.; et al. C9ORF72 Repeat Expansion Causes Vulnerability of Motor Neurons to Ca2+-Permeable AMPA Receptor-Mediated Excitotoxicity. Nat. Commun. 2018, 9, 347. [Google Scholar] [CrossRef]
- Gregory, J.M.; Livesey, M.R.; McDade, K.; Selvaraj, B.T.; Barton, S.K.; Chandran, S.; Smith, C. Dysregulation of AMPA Receptor Subunit Expression in Sporadic ALS Post-mortem Brain. J. Pathol. 2020, 250, 67–78. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Gan, Q.; Salussolia, C.L.; Wollmuth, L.P. Assembly of AMPA Receptors: Mechanisms and Regulation. J. Physiol. 2015, 593, 39–48. [Google Scholar] [CrossRef]
- Isaac, J.T.R.; Ashby, M.C.; McBain, C.J. The Role of the GluR2 Subunit in AMPA Receptor Function and Synaptic Plasticity. Neuron 2007, 54, 859–871. [Google Scholar] [CrossRef]
- Guo, C.; Ma, Y.-Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef]
- Cull-Candy, S.; Kelly, L.; Farrant, M. Regulation of Ca2+-Permeable AMPA Receptors: Synaptic Plasticity and Beyond. Curr. Opin. Neurobiol. 2006, 16, 288–297. [Google Scholar] [CrossRef]
- Grosskreutz, J.; Van Den Bosch, L.; Keller, B.U. Calcium Dysregulation in Amyotrophic Lateral Sclerosis. Cell Calcium 2010, 47, 165–174. [Google Scholar] [CrossRef]
- Hayashi, S.; Amari, M.; Okamoto, K. Loss of Calretinin- and Parvalbumin-Immunoreactive Axons in Anterolateral Columns beyond the Corticospinal Tracts of Amyotrophic Lateral Sclerosis Spinal Cords. J. Neurol. Sci. 2013, 331, 61–66. [Google Scholar] [CrossRef]
- Alexianu, M.E.; Ho, B.; Mohamed, A.H.; La Bella, V.; Smith, R.G.; Appel, S.H. The Role of Calcium-binding Proteins in Selective Motoneuron Vulnerability in Amyotrophic Lateral Sclerosis. Ann. Neurol. 1994, 36, 846–858. [Google Scholar] [CrossRef]
- Siklós, L.; Engelhardt, J.; Harati, Y.; Smith, R.G.; Joó, F.; Appel, S.H. Ultrastructural Evidence for Altered Calcium in Motor Nerve Terminals in Amyotrophc Lateral Sclerosis. Ann. Neurol. 1996, 39, 203–216. [Google Scholar] [CrossRef]
- Couratier, P.; Sindou, P.; Hugon, J.; Couratier, P.; Hugon, J.; Vallat, J.-M.; Dumas, M. Cell Culture Evidence for Neuronal Degeneration in Amyotrophic Lateral Sclerosis Being Linked to Glutamate AMPA/Kainate Receptors. Lancet 1993, 341, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nalini, A.; Laxmi, T.R.; Raju, T.R. ALS-CSF-Induced Structural Changes in Spinal Motor Neurons of Rat Pups Cause Deficits in Motor Behaviour. Exp. Brain Res. 2021, 239, 315–327. [Google Scholar] [CrossRef]
- Spreux-Varoquaux, O.; Bensimon, G.; Lacomblez, L.; Salachas, F.; Pradat, P.F.; Le Forestier, N.; Marouan, A.; Dib, M.; Meininger, V. Glutamate Levels in Cerebrospinal Fluid in Amyotrophic Lateral Sclerosis: A Reappraisal Using a New HPLC Method with Coulometric Detection in a Large Cohort of Patients. J. Neurol. Sci. 2002, 193, 73–78. [Google Scholar] [CrossRef]
- Fiszman, M.L.; Ricart, K.C.; Latini, A.; Rodríguez, G.; Sica, R.E.P. In Vitro Neurotoxic Properties and Excitatory Aminoacids Concentration in the Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Relationship with the Degree of Certainty of Disease Diagnoses. Acta Neurol. Scand. 2010, 121, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Nalini, A.; Joshi, N.B.; Joshi, P.G. Cerebrospinal Fluid from Amyotrophic Lateral Sclerosis Patients Preferentially Elevates Intracellular Calcium and Toxicity in Motor Neurons via AMPA/Kainate Receptor. J. Neurol. Sci. 2005, 235, 45–54. [Google Scholar] [CrossRef]
- Cid, C.; Alvarez-Cermeño, J.C.; Regidor, I.; Salinas, M.; Alcazar, A. Low Concentrations of Glutamate Induce Apoptosis in Cultured Neurons: Implications for Amyotrophic Lateral Sclerosis. J. Neurol. Sci. 2003, 206, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Rembach, A.; Turner, B.J.; Bruce, S.; Cheah, I.K.; Scott, R.L.; Lopes, E.C.; Zagami, C.J.; Beart, P.M.; Cheung, N.S.; Langford, S.J.; et al. Antisense Peptide Nucleic Acid Targeting GluR3 Delays Disease Onset and Progression in the SOD1 G93A Mouse Model of Familial ALS. J. Neurosci. Res. 2004, 77, 573–582. [Google Scholar] [CrossRef]
- Tortarolo, M.; Grignaschi, G.; Calvaresi, N.; Zennaro, E.; Spaltro, G.; Colovic, M.; Fracasso, C.; Guiso, G.; Elger, B.; Schneider, H.; et al. Glutamate AMPA Receptors Change in Motor Neurons of SOD1G93A Transgenic Mice and Their Inhibition by a Noncompetitive Antagonist Ameliorates the Progression of Amytrophic Lateral Sclerosis-like Disease. J. Neurosci. Res. 2006, 83, 134–146. [Google Scholar] [CrossRef]
- Takuma, H.; Kwak, S.; Yoshizawa, T.; Kanazawa, I. Reduction of GluR2 RNA Editing, a Molecular Change That Increases Calcium Influx through AMPA Receptors, Selective in the Spinal Ventral Gray of Patients with Amyotrophic Lateral Sclerosis. Ann. Neurol. 1999, 46, 806–815. [Google Scholar] [CrossRef]
- Kawahara, Y.; Ito, K.; Sun, H.; Aizawa, H.; Kanazawa, I.; Kwak, S. RNA Editing and Death of Motor Neurons. Nature 2004, 427, 801. [Google Scholar] [CrossRef]
- Kawahara, Y.; Kwak, S. Excitotoxicity and ALS: What Is Unique about the AMPA Receptors Expressed on Spinal Motor Neurons? Amyotroph. Lateral Scler. 2005, 6, 131–144. [Google Scholar] [CrossRef]
- Melcher, T.; Maas, S.; Herb, A.; Sprengel, R.; Seeburg, P.H.; Higuchi, M. A Mammalian RNA Editing Enzyme. Nature 1996, 379, 460–464. [Google Scholar] [CrossRef]
- Hideyama, T.; Yamashita, T.; Suzuki, T.; Tsuji, S.; Higuchi, M.; Seeburg, P.H.; Takahashi, R.; Misawa, H.; Kwak, S. Induced Loss of ADAR2 Engenders Slow Death of Motor Neurons from Q/R Site-Unedited GluR2. J. Neurosci. 2010, 30, 11917–11925. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Viscomi, M.T.; Caioli, S.; Pignataro, A.; Bisicchia, E.; Pieri, M.; Molinari, M.; Ammassari-Teule, M.; Zona, C. Altered Functionality, Morphology, and Vesicular Glutamate Transporter Expression of Cortical Motor Neurons from a Presymptomatic Mouse Model of Amyotrophic Lateral Sclerosis. Cereb. Cortex 2016, 26, 1512–1528. [Google Scholar] [CrossRef]
- Jiang, T.; Handley, E.; Brizuela, M.; Dawkins, E.; Lewis, K.E.A.; Clark, R.M.; Dickson, T.C.; Blizzard, C.A. Amyotrophic Lateral Sclerosis Mutant TDP-43 May Cause Synaptic Dysfunction through Altered Dendritic Spine Function. Dis. Model. Mech. 2019, 12, dmm038109. [Google Scholar] [CrossRef]
- Aizawa, H.; Yamashita, T.; Kato, H.; Kimura, T.; Kwak, S. Impaired Nucleoporins Are Present in Sporadic Amyotrophic Lateral Sclerosis Motor Neurons That Exhibit Mislocalization of the 43-KDa TAR DNA-Binding Protein. J. Clin. Neurol. 2019, 15, 62. [Google Scholar] [CrossRef]
- Wobst, H.J.; Mack, K.L.; Brown, D.G.; Brandon, N.J.; Shorter, J. The Clinical Trial Landscape in Amyotrophic Lateral Sclerosis—Past, Present, and Future. Med. Res. Rev. 2020, 40, 1352–1384. [Google Scholar] [CrossRef]
- Pascuzzi, R.M.; Shefner, J.; Chappell, A.S.; Bjerke, J.S.; Tamura, R.; Chaudhry, V.; Clawson, L.; Haas, L.; Rothstein, J.D. A Phase II Trial of Talampanel in Subjects with Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2010, 11, 266–271. [Google Scholar] [CrossRef]
- Akamatsu, M.; Yamashita, T.; Hirose, N.; Teramoto, S.; Kwak, S. The AMPA Receptor Antagonist Perampanel Robustly Rescues Amyotrophic Lateral Sclerosis (ALS) Pathology in Sporadic ALS Model Mice. Sci. Rep. 2016, 6, 28649. [Google Scholar] [CrossRef]
- Turalde, C.W.R.; Moalong, K.M.C.; Espiritu, A.I.; Prado, M.B. Perampanel for Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Neurol. Sci. 2022, 43, 889–897. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Titus, S.; Kearney, M.; Yu, H.; Sherman, A.; Schoenfeld, D.; Hayden, D.; Shui, A.; Brooks, B.; Conwit, R.; et al. Safety and Efficacy of Ceftriaxone for Amyotrophic Lateral Sclerosis: A Multi-Stage, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2014, 13, 1083–1091. [Google Scholar] [CrossRef]
- de Carvalho, M.; Swash, M. Fasciculation Potentials: A Study of Amyotrophic Lateral Sclerosis and Other Neurogenic Disorders. Muscle Nerve 1998, 21, 336–344. [Google Scholar] [CrossRef]
- Trojaborg, W.; Buchthal, F. Malignant and Benign Fasciculations. Acta Neurol. Scand. 2009, 41, 251–254. [Google Scholar] [CrossRef]
- Bashford, J.A.; Wickham, A.; Iniesta, R.; Drakakis, E.M.; Boutelle, M.G.; Mills, K.R.; Shaw, C.E. The Rise and Fall of Fasciculations in Amyotrophic Lateral Sclerosis. Brain Commun. 2020, 2, fcaa018. [Google Scholar] [CrossRef]
- Gelon, P.A.; Dutchak, P.A.; Sephton, C.F. Synaptic Dysfunction in ALS and FTD: Anatomical and Molecular Changes Provide Insights into Mechanisms of Disease. Front. Mol. Neurosci. 2022, 15, 1000183. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Krasnow, E.N.; Fumagalli, L.; Vandoorne, T.; Baatsen, P.; Kerstens, A.; Giacomazzi, G.; Pavie, B.; Rossaert, E.; Beckers, J.; et al. Generation of Human Motor Units with Functional Neuromuscular Junctions in Microfluidic Devices. J. Vis. Exp. 2021, 175, e62959. [Google Scholar] [CrossRef]
- Massih, B.; Veh, A.; Schenke, M.; Mungwa, S.; Seeger, B.; Selvaraj, B.T.; Chandran, S.; Reinhardt, P.; Sterneckert, J.; Hermann, A.; et al. A 3D Cell Culture System for Bioengineering Human Neuromuscular Junctions to Model ALS. Front. Cell Dev. Biol. 2023, 11, 996952. [Google Scholar] [CrossRef]
- Picchiarelli, G.; Demestre, M.; Zuko, A.; Been, M.; Higelin, J.; Dieterlé, S.; Goy, M.-A.; Mallik, M.; Sellier, C.; Scekic-Zahirovic, J.; et al. FUS-Mediated Regulation of Acetylcholine Receptor Transcription at Neuromuscular Junctions Is Compromised in Amyotrophic Lateral Sclerosis. Nat. Neurosci. 2019, 22, 1793–1805. [Google Scholar] [CrossRef]
- Pereira, J.D.; DuBreuil, D.M.; Devlin, A.-C.; Held, A.; Sapir, Y.; Berezovski, E.; Hawrot, J.; Dorfman, K.; Chander, V.; Wainger, B.J. Human Sensorimotor Organoids Derived from Healthy and Amyotrophic Lateral Sclerosis Stem Cells Form Neuromuscular Junctions. Nat. Commun. 2021, 12, 4744. [Google Scholar] [CrossRef]
- Harley, P.; Neves, G.; Riccio, F.; Barcellos Machado, C.; Cheesbrough, A.; R’Bibo, L.; Burrone, J.; Lieberam, I. Pathogenic TDP-43 Disrupts Axon Initial Segment Structure and Neuronal Excitability in a Human IPSC Model of ALS. bioRxiv 2022. [Google Scholar] [CrossRef]
- Krus, K.L.; Strickland, A.; Yamada, Y.; Devault, L.; Schmidt, R.E.; Bloom, A.J.; Milbrandt, J.; DiAntonio, A. Loss of Stathmin-2, a Hallmark of TDP-43-Associated ALS, Causes Motor Neuropathy. Cell Rep. 2022, 39, 111001. [Google Scholar] [CrossRef]
- Chand, K.K.; Lee, K.M.; Lee, J.D.; Qiu, H.; Willis, E.F.; Lavidis, N.A.; Hilliard, M.A.; Noakes, P.G. Defects in Synaptic Transmission at the Neuromuscular Junction Precede Motor Deficits in a TDP-43 Q331K Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. FASEB J. 2018, 32, 2676–2689. [Google Scholar] [CrossRef]
- Gordon, D.; Dafinca, R.; Scaber, J.; Alegre-Abarrategui, J.; Farrimond, L.; Scott, C.; Biggs, D.; Kent, L.; Oliver, P.L.; Davies, B.; et al. Single-Copy Expression of an Amyotrophic Lateral Sclerosis-Linked TDP-43 Mutation (M337V) in BAC Transgenic Mice Leads to Altered Stress Granule Dynamics and Progressive Motor Dysfunction. Neurobiol. Dis. 2019, 121, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Katsuno, M.; Niwa, J.; Takagi, S.; Ishigaki, S.; Ikenaka, K.; Kawai, K.; Watanabe, H.; Yamanaka, K.; Takahashi, R.; et al. Loss of TDP-43 Causes Age-Dependent Progressive Motor Neuron Degeneration. Brain 2013, 136, 1371–1382. [Google Scholar] [CrossRef]
- Holt, C.E.; Martin, K.C.; Schuman, E.M. Local Translation in Neurons: Visualization and Function. Nat. Struct. Mol. Biol. 2019, 26, 557–566. [Google Scholar] [CrossRef]
- Sun, T.; Qiao, H.; Pan, P.-Y.; Chen, Y.; Sheng, Z.-H. Motile Axonal Mitochondria Contribute to the Variability of Presynaptic Strength. Cell Rep. 2013, 4, 413–419. [Google Scholar] [CrossRef]
- López-Erauskin, J.; Tadokoro, T.; Baughn, M.W.; Myers, B.; McAlonis-Downes, M.; Chillon-Marinas, C.; Asiaban, J.N.; Artates, J.; Bui, A.T.; Vetto, A.P.; et al. ALS/FTD-Linked Mutation in FUS Suppresses Intra-Axonal Protein Synthesis and Drives Disease Without Nuclear Loss-of-Function of FUS. Neuron 2018, 100, 816–830.e7. [Google Scholar] [CrossRef]
- Altman, T.; Ionescu, A.; Ibraheem, A.; Priesmann, D.; Gradus-Pery, T.; Farberov, L.; Alexandra, G.; Shelestovich, N.; Dafinca, R.; Shomron, N.; et al. Axonal TDP-43 Condensates Drive Neuromuscular Junction Disruption through Inhibition of Local Synthesis of Nuclear Encoded Mitochondrial Proteins. Nat. Commun. 2021, 12, 6914. [Google Scholar] [CrossRef] [PubMed]
- Baradaran-Heravi, Y.; Van Broeckhoven, C.; van der Zee, J. Stress Granule Mediated Protein Aggregation and Underlying Gene Defects in the FTD-ALS Spectrum. Neurobiol. Dis. 2020, 134, 104639. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Krasnow, E.N.; Fumagalli, L.; Vandoorne, T.; Baatsen, P.; Kerstens, A.; Giacomazzi, G.; Pavie, B.; Rossaert, E.; Beckers, J.; et al. Human Motor Units in Microfluidic Devices Are Impaired by FUS Mutations and Improved by HDAC6 Inhibition. Stem Cell Rep. 2021, 16, 2213–2227. [Google Scholar] [CrossRef]
- Guo, W.; Stoklund Dittlau, K.; Van Den Bosch, L. Axonal Transport Defects and Neurodegeneration: Molecular Mechanisms and Therapeutic Implications. Semin. Cell Dev. Biol. 2020, 99, 133–150. [Google Scholar] [CrossRef]
- Altman, T.; Geller, D.; Kleeblatt, E.; Gradus-Perry, T.; Perlson, E. An In Vitro Compartmental System Underlines the Contribution of Mitochondrial Immobility to the ATP Supply in the NMJ. J. Cell Sci. 2019, 132, jcs234492. [Google Scholar] [CrossRef]
- Lobsiger, C.S.; Cleveland, D.W. Glial Cells as Intrinsic Components of Non-Cell-Autonomous Neurodegenerative Disease. Nat. Neurosci. 2007, 10, 1355–1360. [Google Scholar] [CrossRef]
- Yamanaka, K.; Chun, S.J.; Boillee, S.; Fujimori-Tonou, N.; Yamashita, H.; Gutmann, D.H.; Takahashi, R.; Misawa, H.; Cleveland, D.W. Astrocytes as Determinants of Disease Progression in Inherited Amyotrophic Lateral Sclerosis. Nat. Neurosci. 2008, 11, 251–253. [Google Scholar] [CrossRef]
- Boillée, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science (1979) 2006, 312, 1389–1392. [Google Scholar] [CrossRef]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and Impaired Regeneration of Gray Matter Oligodendrocytes in Amyotrophic Lateral Sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Sullivan, C.R.; McCullumsmith, R.E. The Role of Glutamate Transporters in the Pathophysiology of Neuropsychiatric Disorders. NPJ Schizophr. 2017, 3, 32. [Google Scholar] [CrossRef]
- Todd, A.C.; Hardingham, G.E. The Regulation of Astrocytic Glutamate Transporters in Health and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9607. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Van Kammen, M.; Levey, A.I.; Martin, L.J.; Kuncl, R.W. Selective Loss of Glial Glutamate Transporter GLT-1 in Amyotrophic Lateral Sclerosis. Ann. Neurol. 1995, 38, 73–84. [Google Scholar] [CrossRef]
- Rothstein, J.D. Therapeutic Horizons for Amyotrophic Lateral Sclerosis. Curr. Opin. Neurobiol. 1996, 6, 679–687. [Google Scholar] [CrossRef]
- Allen, N.J.; Bennett, M.L.; Foo, L.C.; Wang, G.X.; Chakraborty, C.; Smith, S.J.; Barres, B.A. Astrocyte Glypicans 4 and 6 Promote Formation of Excitatory Synapses via GluA1 AMPA Receptors. Nature 2012, 486, 410–414. [Google Scholar] [CrossRef]
- Kia, A.; McAvoy, K.; Krishnamurthy, K.; Trotti, D.; Pasinelli, P. Astrocytes Expressing ALS-Linked Mutant FUS Induce Motor Neuron Death through Release of Tumor Necrosis Factor-Alpha. Glia 2018, 66, 1016–1033. [Google Scholar] [CrossRef]
- Nagai, M.; Re, D.B.; Nagata, T.; Chalazonitis, A.; Jessell, T.M.; Wichterle, H.; Przedborski, S. Astrocytes Expressing ALS-Linked Mutated SOD1 Release Factors Selectively Toxic to Motor Neurons. Nat. Neurosci. 2007, 10, 615–622. [Google Scholar] [CrossRef]
- Díaz-Amarilla, P.; Olivera-Bravo, S.; Trias, E.; Cragnolini, A.; Martínez-Palma, L.; Cassina, P.; Beckman, J.; Barbeito, L. Phenotypically Aberrant Astrocytes That Promote Motoneuron Damage in a Model of Inherited Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 18126–18131. [Google Scholar] [CrossRef]
- Zhao, C.; Devlin, A.; Chouhan, A.K.; Selvaraj, B.T.; Stavrou, M.; Burr, K.; Brivio, V.; He, X.; Mehta, A.R.; Story, D.; et al. Mutant C9orf72 Human IPSC-derived Astrocytes Cause Non-cell Autonomous Motor Neuron Pathophysiology. Glia 2020, 68, 1046–1064. [Google Scholar] [CrossRef]
- Olsen, M.L.; Sontheimer, H. Functional Implications for Kir4.1 Channels in Glial Biology: From K + Buffering to Cell Differentiation. J. Neurochem. 2008, 107, 589–601. [Google Scholar] [CrossRef]
- Kinboshi, M.; Ikeda, A.; Ohno, Y. Role of Astrocytic Inwardly Rectifying Potassium (Kir) 4.1 Channels in Epileptogenesis. Front. Neurol. 2020, 11, 626658. [Google Scholar] [CrossRef]
- Kelley, K.W.; Ben Haim, L.; Schirmer, L.; Tyzack, G.E.; Tolman, M.; Miller, J.G.; Tsai, H.-H.; Chang, S.M.; Molofsky, A.V.; Yang, Y.; et al. Kir4.1-Dependent Astrocyte-Fast Motor Neuron Interactions Are Required for Peak Strength. Neuron 2018, 98, 306–319.e7. [Google Scholar] [CrossRef]
- Tripathi, P.; Rodriguez-Muela, N.; Klim, J.R.; de Boer, A.S.; Agrawal, S.; Sandoe, J.; Lopes, C.S.; Ogliari, K.S.; Williams, L.A.; Shear, M.; et al. Reactive Astrocytes Promote ALS-like Degeneration and Intracellular Protein Aggregation in Human Motor Neurons by Disrupting Autophagy through TGF-Β1. Stem Cell Rep. 2017, 9, 667–680. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Terrie, L.; Baatsen, P.; Kerstens, A.; De Swert, L.; Janky, R.; Corthout, N.; Masrori, P.; Van Damme, P.; Hyttel, P.; et al. FUS-ALS HiPSC-Derived Astrocytes Impair Human Motor Units through Both Gain-of-Toxicity and Loss-of-Support Mechanisms. Mol. Neurodegener. 2023, 18, 5. [Google Scholar] [CrossRef]
- Vahsen, B.F.; Nalluru, S.; Morgan, G.R.; Farrimond, L.; Carroll, E.; Xu, Y.; Cramb, K.M.L.; Amein, B.; Scaber, J.; Katsikoudi, A.; et al. C9orf72-ALS Human IPSC Microglia Are pro-Inflammatory and Toxic to Co-Cultured Motor Neurons via MMP9. Nat. Commun. 2023, 14, 5898. [Google Scholar] [CrossRef]
- Banerjee, P.; Mehta, A.R.; Nirujogi, R.S.; Cooper, J.; James, O.G.; Nanda, J.; Longden, J.; Burr, K.; McDade, K.; Salzinger, A.; et al. Cell-Autonomous Immune Dysfunction Driven by Disrupted Autophagy in C9orf72 -ALS IPSC-Derived Microglia Contributes to Neurodegeneration. Sci. Adv. 2023, 9, eabq0651. [Google Scholar] [CrossRef]
- Petrov, D.; Mansfield, C.; Moussy, A.; Hermine, O. ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment? Front. Aging Neurosci. 2017, 9, 68. [Google Scholar] [CrossRef]
- Gu, Z.; Kalambogias, J.; Yoshioka, S.; Han, W.; Li, Z.; Kawasawa, Y.I.; Pochareddy, S.; Li, Z.; Liu, F.; Xu, X.; et al. Control of Species-Dependent Cortico-Motoneuronal Connections Underlying Manual Dexterity. Science (1979) 2017, 357, 400–404. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved Cell Types with Divergent Features in Human versus Mouse Cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Jones, R.A.; Harrison, C.; Eaton, S.L.; Llavero Hurtado, M.; Graham, L.C.; Alkhammash, L.; Oladiran, O.A.; Gale, A.; Lamont, D.J.; Simpson, H.; et al. Cellular and Molecular Anatomy of the Human Neuromuscular Junction. Cell Rep. 2017, 21, 2348–2356. [Google Scholar] [CrossRef]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In Vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Denis, H.L.; Cicchetti, F.; Alpaugh, M. Current and Future Applications of Induced Pluripotent Stem Cell-Based Models to Study Pathological Proteins in Neurodegenerative Disorders. Mol. Psychiatry 2021, 26, 2685–2706. [Google Scholar] [CrossRef]
- Qian, X.; Song, H.; Ming, G. Brain Organoids: Advances, Applications and Challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef]
- Buchner, F.; Dokuzluoglu, Z.; Grass, T.; Rodriguez-Muela, N. Spinal Cord Organoids to Study Motor Neuron Development and Disease. Life 2023, 13, 1254. [Google Scholar] [CrossRef]
- Andersen, J.; Revah, O.; Miura, Y.; Thom, N.; Amin, N.D.; Kelley, K.W.; Singh, M.; Chen, X.; Thete, M.V.; Walczak, E.M.; et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 2020, 183, 1913–1929.e26. [Google Scholar] [CrossRef]
- Miura, Y.; Li, M.-Y.; Revah, O.; Yoon, S.-J.; Narazaki, G.; Pașca, S.P. Engineering Brain Assembloids to Interrogate Human Neural Circuits. Nat. Protoc. 2022, 17, 15–35. [Google Scholar] [CrossRef]
- Andrews, M.G.; Kriegstein, A.R. Challenges of Organoid Research. Annu. Rev. Neurosci. 2022, 45, 23–39. [Google Scholar] [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia Integration into Human Midbrain Organoids Leads to Increased Neuronal Maturation and Functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of Human Brain Organoids with a Functional Vascular-like System. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Shirure, V.S.; Hughes, C.C.W.; George, S.C. Engineering Vascularized Organoid-on-a-Chip Models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Cakir, B.; Patterson, B.; Kim, K.-Y.; Sun, P.; Kang, Y.-J.; Zhong, M.; Liu, X.; Patra, P.; et al. HESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 2019, 24, 487–497.e7. [Google Scholar] [CrossRef]
- Reumann, D.; Krauditsch, C.; Novatchkova, M.; Sozzi, E.; Wong, S.N.; Zabolocki, M.; Priouret, M.; Doleschall, B.; Ritzau-Reid, K.I.; Piber, M.; et al. In Vitro Modeling of the Human Dopaminergic System Using Spatially Arranged Ventral Midbrain–Striatum–Cortex Assembloids. Nat. Methods 2023, 20, 2034–2047. [Google Scholar] [CrossRef]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused Cerebral Organoids Model Interactions between Brain Regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Bowles, K.R.; Silva, M.C.; Whitney, K.; Bertucci, T.; Berlind, J.E.; Lai, J.D.; Garza, J.C.; Boles, N.C.; Mahali, S.; Strang, K.H.; et al. ELAVL4, Splicing, and Glutamatergic Dysfunction Precede Neuron Loss in MAPT Mutation Cerebral Organoids. Cell 2021, 184, 4547–4563.e17. [Google Scholar] [CrossRef] [PubMed]
- Faustino Martins, J.-M.; Fischer, C.; Urzi, A.; Vidal, R.; Kunz, S.; Ruffault, P.-L.; Kabuss, L.; Hube, I.; Gazzerro, E.; Birchmeier, C.; et al. Self-Organizing 3D Human Trunk Neuromuscular Organoids. Cell Stem Cell 2020, 26, 172–186.e6. [Google Scholar] [CrossRef] [PubMed]
- James, O.G.; Selvaraj, B.T.; Magnani, D.; Burr, K.; Connick, P.; Barton, S.K.; Vasistha, N.A.; Hampton, D.W.; Story, D.; Smigiel, R.; et al. IPSC-Derived Myelinoids to Study Myelin Biology of Humans. Dev. Cell 2021, 56, 1346–1358.e6. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzinger, A.; Ramesh, V.; Das Sharma, S.; Chandran, S.; Thangaraj Selvaraj, B. Neuronal Circuit Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2024, 13, 792. https://doi.org/10.3390/cells13100792
Salzinger A, Ramesh V, Das Sharma S, Chandran S, Thangaraj Selvaraj B. Neuronal Circuit Dysfunction in Amyotrophic Lateral Sclerosis. Cells. 2024; 13(10):792. https://doi.org/10.3390/cells13100792
Chicago/Turabian StyleSalzinger, Andrea, Vidya Ramesh, Shreya Das Sharma, Siddharthan Chandran, and Bhuvaneish Thangaraj Selvaraj. 2024. "Neuronal Circuit Dysfunction in Amyotrophic Lateral Sclerosis" Cells 13, no. 10: 792. https://doi.org/10.3390/cells13100792





