The Importance of HHLA2 in Solid Tumors—A Review of the Literature
Abstract
:1. Introduction
2. B7 Family
3. HHLA2 Structure and Expression
| Type of Tumor | Expression Rate | HHLA-2 Expression and Prognosis | Potential Mechanisms | References |
|---|---|---|---|---|
| colorectal cancer | 83.7% | Unclear | Unclear—potential co-stimulatory and co-inhibitory effects; EMT and cell cycle regulation | [23,24,25] |
| gastric cancer | 53.2% (high expression) | Unfavorable prognosis in the case of elevated HHLA-2 expression in tumor tissues | Unclear | [26] |
| renal cancer | 94.57% | Unclear | EMT regulation | [12,27,28,29,30] |
| hepatic cancer | 49.0%–67.7% | Unfavorable prognosis in the case of elevated HHLA-2 expression in tumor tissues | Inhibitory effect on immunological responses | [13,31,32,33,34,35] |
| gallbladder cancer | 53.68%–100% | Unfavorable prognosis in the case of elevated HHLA-2 expression in tumor tissues | EMT promotion | [36,37] |
| pancreatic cancer | 77% | More favorable prognosis in the case of HHLA-2 expression in tumor tissues | Stimulation of immunological responses | [38,39] |
| lung cancer | 11.1% (NSCLC); 68.6% (adenocarcinoma) | Unfavorable prognosis in the case of elevated HHLA-2 expression in tumor tissues | Cell cycle and EMT regulation | [19,38,40,41] |
| ovarian cancer | 17.19%–100% | Unfavorable prognosis in the case of elevated HHLA-2 expression in the stromal compartment | Unclear | [42,43] |
| breast cancer | 56% (upregulation) | Unclear | Unclear | [44,45] |
| urothelial cancer | 77.8% | UTUC—more favorable prognosis in the case of increased HHLA-2 expression in tumor cells; BUC—unfavorable prognosis in the case of elevated HHLA-2 expression | Unclear | [46,47] |
| thyroid cancer | 100% | Unfavorable prognosis in the case of elevated HHLA-2 expression in tumor tissues | Inhibition of immunological responses | [48,49] |
| cervical cancer | 97.4% | More favorable prognosis in the case of increased HHLA-2 expression in tumor tissues | Unclear | [50] |
| Type of Tumor | HHLA2 Expression and Immune Infiltration | References | |
|---|---|---|---|
| Increase in | Decrease in | ||
| colorectal cancer | CD4+ resting cells, activated dendritic cells, eosinophils | CD8+ T cells, NK resting cells, M0 macrophages | [23,25] |
| renal cancer | CD8+ cells | - | [12] |
| hepatic cancer | CD4+ Foxp3+ cells (ICC), M0 macrophages, neutrophils, follicular helper T cells, memory-activated CD4+ T cells, Tregs, dendritic cells (HCC) (unclear) | CD3+, CD8+ cells (ICC), M2 macrophages, monocytes, activated mast cells, NK cells (HCC), CD4+, CD8+ cells, Tregs (HCC) (unclear) | [13,31,33,35] |
| gallbladder cancer | - | CD8+ cells | [37] |
| pancreatic cancer | CD8+ cells | - | [38] |
| lung cancer | M2 macrophages (adenocarcinoma) | - | [19] |
| ovarian cancer | CD8+ cells | - | [43] |
| thyroid cancer | - | CD8+ cells | [49] |
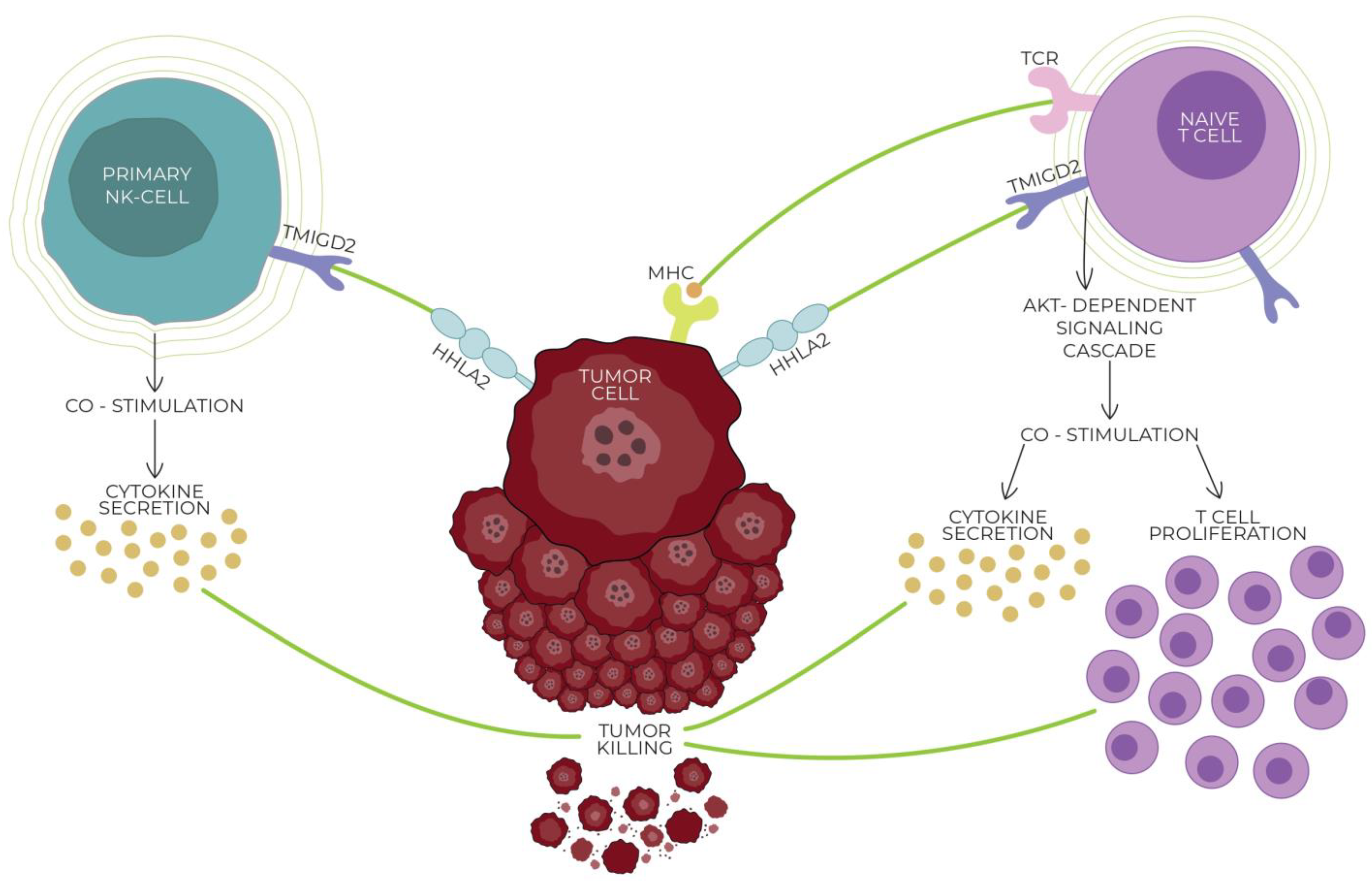
4. HHLA2 Receptors—Immunosuppression and Immunostimulation
5. HHLA2 vs. Hot and Cold Tumors
6. HHLA2 and Cancer Stem Cells
7. HHLA2 in Different Malignancies
7.1. HHLA2 in Colorectal Cancer
7.2. HHLA2 in Gastric Cancer
7.3. HHLA2 in Renal Cancer
7.4. HHLA2 in Hepatic Cancer
7.5. HHLA2 in Gallbladder Cancer
7.6. HHLA2 in Pancreatic Cancer
7.7. HHLA2 in Oral and Esophageal Cancer
7.8. HHLA2 in Lung Cancer
7.9. HHLA2 in Ovarian Cancer
7.10. HHLA2 in Cervical Cancer
7.11. HHLA2 and Breast Cancer
7.12. HHLA2 in Urothelial Cancer
7.13. HHLA2 in Thyroid Cancer
8. Future Directions
9. Conclusions
Funding
Conflicts of Interest
References
- Qi, Y.; Deng, G.; Xu, P.; Zhang, H.; Yuan, F.; Geng, R.; Jiang, H.; Liu, B.; Chen, Q. HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol. Rep. 2019, 42, 2309–2322. [Google Scholar] [CrossRef]
- Szeto, G.L.; Finley, S.D. Integrative Approaches to Cancer Immunotherapy. Trends Cancer 2019, 5, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Yang, Y.; Weng, L.; Wu, Q.; Zhang, J.; Zhao, P.; Fang, L.; Shi, Y.; Wang, P. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Kallingal, A.; Olszewski, M.; Maciejewska, N.; Brankiewicz, W.; Baginski, M. Cancer immune escape: The role of antigen presentation machinery. J. Cancer Res. Clin. Oncol. 2023, 149, 8131–8141. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef] [PubMed]
- Shimu, A.S.; Wei, H.-X.; Li, Q.; Zheng, X.; Li, B. The new progress in cancer immunotherapy. Clin. Exp. Med. 2023, 23, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, R.; Debnath, D.; Hartley, M.L.; Noel, M.S. The Role of Immunotherapy in Pancreatic Cancer. Curr. Oncol. 2022, 29, 6864–6892. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.H.; Byrne, K.T.; Vonderheide, R.H. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer 2018, 4, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, D. HHLA2 Immune Checkpoint Is a Novel Prognostic Predictor in Hepatocellular Carcinoma. Am. J. Clin. Pathol. 2022, 158, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-X.; Wu, J.-W.; Cheng, X.-Q.; Wang, J.-H.; Wen, X.-Z.; Li, J.-J.; Zhang, Q.; Jiang, H.; Ding, Q.-Y.; Zhu, X.-F.; et al. HHLA2 predicts improved prognosis of anti-PD-1/PD-L1 immunotherapy in patients with melanoma. Front. Immunol. 2022, 13, 902167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, C.; Yu, Y.; Wu, B.; Zhang, Y.; Lang, Q.; Liang, Z.; Zhong, C.; Shi, Y.; Han, S.; et al. KIR3DL3-HHLA2 and TMIGD2-HHLA2 pathways: The dual role of HHLA2 in immune responses and its potential therapeutic approach for cancer immunotherapy. J. Adv. Res. 2023, 47, 137–150. [Google Scholar] [CrossRef]
- Zhou, Q.-H.; Li, K.-W.; Chen, X.; He, H.-X.; Peng, S.-M.; Peng, S.-R.; Wang, Q.; Li, Z.-A.; Tao, Y.-R.; Cai, W.-L.; et al. HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.-Y.; Fu, Y.-P.; Yi, Y.; Zhang, M.-X.; Zheng, S.-S.; Huang, J.-L.; Gan, W.; Xu, X.; Lin, J.-J.; Zhang, J.; et al. HHLA2 in intrahepatic cholangiocarcinoma: An immune checkpoint with prognostic significance and wider expression compared with PD-L1. J. Immunother. Cancer 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Bolandi, N.; Derakhshani, A.; Hemmat, N.; Baghbanzadeh, A.; Asadzadeh, Z.; Afrashteh Nour, M.; Brunetti, O.; Bernardini, R.; Silvestris, N.; Baradaran, B. The Positive and Negative Immunoregulatory Role of B7 Family: Promising Novel Targets in Gastric Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10719. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Xia, Y.; Wang, Y.; Wang, Y.; Shi, Y.; Xing, H.; Qu, T.; Wang, Y.; Ma, W. Immune checkpoint of B7-H3 in cancer: From immunology to clinical immunotherapy. J. Hematol. Oncol. 2022, 15, 153. [Google Scholar] [CrossRef]
- Zhao, R.; Chinai, J.M.; Buhl, S.; Scandiuzzi, L.; Ray, A.; Jeon, H.; Ohaegbulam, K.C.; Ghosh, K.; Zhao, A.; Scharff, M.D.; et al. HHLA2 is a member of the B7 family and inhibits human CD4+ and CD8 T-cell function. Proc. Natl. Acad. Sci. USA 2013, 110, 9879–9884. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. HHLA2 immune-regulatory roles in cancer. Biomed. Pharmacother. 2023, 162, 114639. [Google Scholar] [CrossRef]
- Wei, Y.; Ren, X.; Galbo, P.M.; Moerdler, S.; Wang, H.; Sica, R.A.; Etemad-Gilbertson, B.; Shi, L.; Zhu, L.; Tang, X.; et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci. Immunol. 2021, 6, eabf9792. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, S.; Tang, G.; Sun, S.; Luo, Y.; Bai, R.; Han, L.; Jiang, X.; Gao, Y.; Huang, Z.; et al. HHLA2 deficiency inhibits non-small cell lung cancer progression and THP-1 macrophage M2 polarization. Cancer Med. 2021, 10, 5256–5269. [Google Scholar] [CrossRef]
- Cheng, H.; Borczuk, A.; Janakiram, M.; Ren, X.; Lin, J.; Assal, A.; Halmos, B.; Perez-Soler, R.; Zang, X. Wide Expression and Significance of Alternative Immune Checkpoint Molecules, B7x and HHLA2, in PD-L1-Negative Human Lung Cancers. Clin. Cancer Res. 2018, 24, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Dong, W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco. Targets. Ther. 2018, 11, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Cao, L.; Li, Y. A novel 10-gene immune-related lncRNA signature model for the prognosis of colorectal cancer. Math. Biosci. Eng. 2021, 18, 9743–9760. [Google Scholar] [CrossRef] [PubMed]
- Kula, A.; Dawidowicz, M.; Mielcarska, S.; Kiczmer, P.; Skiba, H.; Krygier, M.; Chrabańska, M.; Piecuch, J.; Szrot, M.; Robotycka, J.; et al. Overexpression and Role of HHLA2, a Novel Immune Checkpoint, in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 5876. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tang, L.; Chang, H.; Huo, S.; Li, Y. HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Hum. Cell 2020, 33, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, W.; Xu, Y.; Zhu, M.; Xiao, Y.; Shen, Y.; Zhu, S.; Cao, C.; Xu, X. Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: A novel prognostic biomarker and potential therapeutic target. J. Med. Genet. 2019, 56, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, D.; Feng, J.; Zhou, Y.; Wang, Q.; Feng, H.; Zhang, J.; Jiang, J. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wan, Y.; Yang, M.; Qi, X.; Dong, Z.; Huang, J.; Xu, J. Identification of methylation-driven genes related to the prognosis of papillary renal cell carcinoma: A study based on The Cancer Genome Atlas. Cancer Cell Int. 2020, 20, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Hu, X. Identification and Comprehensive Validation of a DNA Methylation-Driven Gene-Based Prognostic Model for Clear Cell Renal Cell Carcinoma. DNA Cell Biol. 2020, 39, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, H.; Tang, X.; Zhang, T.; Liu, Y.; Zhang, C.; Yu, H.; Li, Y. Interferon Gamma-Induced Interferon Regulatory Factor 1 Activates Transcription of HHLA2 and Induces Immune Escape of Hepatocellular Carcinoma Cells. Inflammation 2022, 45, 308–330. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, Y.; Liang, R.; Li, Y.; Ge, L. Clinical Significance of the HHLA2 Protein in Hepatocellular Carcinoma and the Tumor Microenvironment. J. Inflamm. Res. 2021, 14, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, C.; Tang, X.; Zhang, T.; Liu, Y.; Yu, H.; Li, Y.; Wang, R. HHLA2 Activates the JAK/STAT Signaling Pathway by Binding to TMIGD2 in Hepatocellular Carcinoma Cells. Inflammation 2022, 45, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Z.; Yu, X.; Li, Z.; Zheng, L.; Xu, J. HHLA2 Expression is Associated with Poor Survival in Patients with Hepatocellular Carcinoma. Biologics 2021, 15, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yu, Q.; Yang, S.; Yang, W.-J.; Liu, T.; Xian, J.-R.; Tian, T.-T.; Li, T.; Chen, W.; Wang, B.-L.; et al. Comprehensive Analysis of HHLA2 as a Prognostic Biomarker and Its Association With Immune Infiltrates in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 831101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Lv, C.; Wu, B.; Yu, Y.; Zhong, C.; Lang, Q.; Liang, Z.; Li, Y.; Shi, Y.; et al. HHLA2 promotes tumor progression by long non-coding RNA H19 in human gallbladder cancer. Int. J. Oncol. 2022, 61, 112. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Han, S.; Wu, B.; Liang, Z.; Li, Y.; Zhang, Y.; Lang, Q.; Zhong, C.; Fu, L.; Yu, Y.; et al. Novel immune scoring dynamic nomograms based on B7-H3, B7-H4, and HHLA2: Potential prediction in survival and immunotherapeutic efficacy for gallbladder cancer. Front. Immunol. 2022, 13, 984172. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.P.C.; Sideras, K.; Biermann, K.; Hosein Aziz, M.; Levink, I.J.M.; Mancham, S.; Erler, N.S.; Tang, X.; van Eijck, C.H.; Bruno, M.J.; et al. HHLA2 is expressed in pancreatic and ampullary cancers and increased expression is associated with better post-surgical prognosis. Br. J. Cancer 2020, 122, 1211–1218. [Google Scholar] [CrossRef]
- Yan, H.; Qiu, W.; Koehne de Gonzalez, A.K.; Wei, J.-S.; Tu, M.; Xi, C.-H.; Yang, Y.-R.; Peng, Y.-P.; Tsai, W.-Y.; Remotti, H.E.; et al. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett. 2019, 442, 333–340. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, R.; Li, X.; Shi, Z.; Tian, H.; Feng, J.; Yu, S. B7-H4 and HHLA2, members of B7 family, are aberrantly expressed in EGFR mutated lung adenocarcinoma. Pathol. Res. Pract. 2020, 216, 153134. [Google Scholar] [CrossRef] [PubMed]
- Farrag, M.S.; Ibrahim, E.M.; El-Hadidy, T.A.; Akl, M.F.; Elsergany, A.R.; Abdelwahab, H.W. Human Endogenous Retrovirus-H Long Terminal Repeat- Associating Protein 2 (HHLA2) is a Novel Immune Checkpoint Protein in Lung Cancer which Predicts Survival. Asian Pac. J. Cancer Prev. 2021, 22, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ding, Y.; Liu, J.; Zheng, X.; Wei, W.; Ying, Y.; Wu, C.; Jiang, J.; Ju, J. B7-H7 is a prognostic biomarker in epithelial ovarian cancer. Transl. Cancer Res. 2020, 9, 5360–5370. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shi, Y.; Ling, X.; Wang, D.; Liu, Y.; Lu, H.; Peng, Y.; Zhang, B. HHLA2 predicts better survival and exhibits inhibited proliferation in epithelial ovarian cancer. Cancer Cell Int. 2021, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, M.; Chinai, J.M.; Fineberg, S.; Fiser, A.; Montagna, C.; Medavarapu, R.; Castano, E.; Jeon, H.; Ohaegbulam, K.C.; Zhao, R.; et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin. Cancer Res. 2015, 21, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, H.; Jin, Y.; Yu, Y.; Wang, Y.; Wu, D.; Guo, Y.; Xi, L.; Ye, D.; Pan, Y.; et al. Analysis of a new therapeutic target and construction of a prognostic model for breast cancer based on ferroptosis genes. Comput. Biol. Med. 2023, 165, 107370. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ye, H.; Wang, J.; Chen, S.; Chen, X.; Zhang, C. Immune Checkpoint Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 is Upregulated and Independently Predicts Unfavorable Prognosis in Bladder Urothelial Carcinoma. Nephron 2019, 141, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, D.; Kijima, T.; Arai, K.; Kamai, T. Increased co-expression of stromal HHLA2 and fibroblast activation protein in upper tract urothelial carcinoma. Int. Urol. Nephrol. 2023, 55, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Huang, Y.; Dong, A.; Sun, Y. Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 Possesses Prognostic Significance and Promotes Progression of Papillary Thyroid Cancer. Int. J. Gen. Med. 2022, 15, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, W.; Jiang, X.; Huang, Y.; Yan, S.; Jiang, Y. High expression of HHLA2 predicts poor prognosis in medullary thyroid carcinoma. Jpn. J. Clin. Oncol. 2022, 52, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Cho, H.J.; Park, H.Y.; Lee, D.S.; Choi, I.H.; Kim, Y.N.; Jeong, C.H.; Kim, D.H.; Hwa Im, D.; Min, B.J.; et al. The clinical significance of HERV-H LTR -associating 2 expression in cervical adenocarcinoma. Medicine 2021, 100, e23691. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.S.; Berjis, A.; Konge, J.C.; Mahoney, K.M.; Klee, A.N.; Freeman, S.S.; Chen, C.-H.; Jegede, O.A.; Catalano, P.J.; Pignon, J.-C.; et al. KIR3DL3 Is an Inhibitory Receptor for HHLA2 that Mediates an Alternative Immunoinhibitory Pathway to PD1. Cancer Immunol. Res. 2021, 9, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, M.; Chinai, J.M.; Zhao, A.; Sparano, J.A.; Zang, X. HHLA2 and TMIGD2: New immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology 2015, 4, e1026534. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.H.; Leaton, L.A.; Campos Codo, A.; Crute, B.; Roest, J.; Zhu, S.; Petersen, J.; Tobin, R.P.; Hume, P.S.; Stone, M.; et al. Polymorphic KIR3DL3 expression modulates tissue-resident and innate-like T cells. Sci. Immunol. 2023, 8, eade5343. [Google Scholar] [CrossRef] [PubMed]
- Nutalai, R.; Gaudieri, S.; Jumnainsong, A.; Leelayuwat, C. Regulation of KIR3DL3 Expression via Mirna. Genes 2019, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm 2023, 4, e343. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. High tumor mutation burden (TMB) in microsatellite stable (MSS) colorectal cancers: Diverse molecular associations point to variable pathophysiology. Cancer Treat. Res. Commun. 2023, 36, 100746. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, D.; Ye, X.; Lin, X.; Zhang, M.; Su, Y.; Xie, Q.; Ni, W. HHLA2 Used as a Potential Prognostic and Immunological Biomarker and Correlated with Tumor Microenvironment in Pan-Cancer. Biomed Res. Int. 2022, 2022, 3924400. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Lee, H.; Kim, D.G.; Kim, H.; Ha, S.Y.; Choi, Y.-L.; Jang, K.-T.; Kim, K.-M. PD-L1 Expression Is Significantly Associated with Tumor Mutation Burden and Microsatellite Instability Score. Cancers 2021, 13, 4659. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Albacker, L.A.; Hopkins, A.C.; Montesion, M.; Murugesan, K.; Vithayathil, T.T.; Zaidi, N.; Azad, N.S.; Laheru, D.A.; Frampton, G.M.; et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019, 4, e126908. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Puglisi, S.; Pirrone, C.; Martelli, V.; Catalano, F.; Nardin, S.; Seeber, A.; Puccini, A.; Sciallero, S. The role of immunotherapy in microsatellites stable metastatic colorectal cancer: State of the art and future perspectives. Front. Oncol. 2023, 13, 1161048. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.H.; Ciombor, K.K.; Diaz, L.A.; Yu, J.; Kim, R. Immunotherapy for Microsatellite Stable Colorectal Cancers: Challenges and Novel Therapeutic Avenues. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 242–253. [Google Scholar] [CrossRef]
- Ciurea, M.E.; Georgescu, A.M.; Purcaru, S.O.; Artene, S.-A.; Emami, G.H.; Boldeanu, M.V.; Tache, D.E.; Dricu, A. Cancer stem cells: Biological functions and therapeutically targeting. Int. J. Mol. Sci. 2014, 15, 8169–8185. [Google Scholar] [CrossRef] [PubMed]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J.; Saya, H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016, 107, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hoque, M.O. Targeting Cancer Stem Cells: A Strategy for Effective Eradication of Cancer. Cancers 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Xiong, Y.; Lin, Y.; Liang, R.; Li, Y.; Ge, L. H Long Terminal Repeat-Associating 2 (HHLA2) is a Biomarker of Advanced Stage Hepatocellular Carcinoma and Promotes Tumor Cell Development In Vitro. Med. Sci. Monit. 2021, 27, e930215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Acuna-Villaorduna, A.; Kuan, K.; Gupta, S.; Hu, S.; Ohaegbulam, K.; Albanese, J.; Kaumaya, M.; Levy, R.; Hwang, R.R.; et al. B7-H3 and PD-L1 Expression Are Prognostic Biomarkers in a Multi-racial Cohort of Patients with Colorectal Cancer. Clin. Colorectal Cancer 2021, 20, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Gawesh, Z.M.; Ibrahim, E.M.; ElKalla, H.M.H.R.; Awad, A.A.H.; Mohamed, M.A. Evaluation of HHLA2 and CD8 Immunohistochemical Expression in Colorectal Carcinoma and Their Prognostic Significance. Asian Pac. J. Cancer Prev. 2023, 24, 4309–4319. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shen, J.; Qiao, X.; Gao, Y.; Su, H.-B.; Zhang, S. Long Non-Coding RNA Signatures Associated with Ferroptosis Predict Prognosis in Colorectal Cancer. Int. J. Gen. Med. 2022, 15, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Shimonosono, M.; Arigami, T.; Yanagita, S.; Matsushita, D.; Uchikado, Y.; Kijima, Y.; Kurahara, H.; Kita, Y.; Mori, S.; Sasaki, K.; et al. The association of human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2) expression with gastric cancer prognosis. Oncotarget 2018, 9, 22069–22078. [Google Scholar] [CrossRef] [PubMed]
- Mansorunov, D.; Apanovich, N.; Kipkeeva, F.; Nikulin, M.; Malikhova, O.; Stilidi, I.; Karpukhin, A. The Correlation of Ten Immune Checkpoint Gene Expressions and Their Association with Gastric Cancer Development. Int. J. Mol. Sci. 2022, 23, 13846. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Zhang, C.; Li, F.; Li, L.; Wang, D.; Chand, D.; Guan, F.; Zang, X.; Zhang, Y. Over-Expression and Prognostic Significance of HHLA2, a New Immune Checkpoint Molecule, in Human Clear Cell Renal Cell Carcinoma. Front. Cell Dev. Biol. 2020, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Terrematte, P.; Andrade, D.S.; Justino, J.; Stransky, B.; de Araújo, D.S.A.; Dória Neto, A.D. A Novel Machine Learning 13-Gene Signature: Improving Risk Analysis and Survival Prediction for Clear Cell Renal Cell Carcinoma Patients. Cancers 2022, 14, 2111. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, T.; Perrot, N.; Huang, Z.; Bhatt, R.S.; Sheshdeh, A.B.; Ahmar, N.E.; Ghandour, F.; Signoretti, S.; McDermott, D.F.; Freeman, G.J.; et al. Regulation of HHLA2 expression in kidney cancer and myeloid cells. BMC Cancer 2023, 23, 1039. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Xu, J.; Chen, L.; Xu, C.; Chen, F.; Xu, Z.; Zhang, Y.; Xia, S.; Shao, Y.; et al. Ferroptosis-related gene CHAC1 is a valid indicator for the poor prognosis of kidney renal clear cell carcinoma. J. Cell. Mol. Med. 2021, 25, 3610–3621. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Vinod, P.K. Integrative analysis of DNA methylation and gene expression in papillary renal cell carcinoma. Mol. Genet. Genomics 2020, 295, 807–824. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Sun, X.; Li, K.; Zhang, Y.; Zuo, S.; Li, C.; Wan, S.; Huang, D. Construction and Validation of a Novel Immune Checkpoint-Related Model in Clear Cell Renal Cell Carcinoma. Dis. Markers 2022, 2022, 9010514. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-Y.; Yang, Z.-F.; Wang, Z.-T.; Liu, G.; Zhou, C.; Zhou, J.; Fan, J.; Gan, W.; Yi, Y.; Qiu, S.-J. Integrative analyses identify CD73 as a prognostic biomarker and immunotherapeutic target in intrahepatic cholangiocarcinoma. World J. Surg. Oncol. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, M.; Yu, J.; Hu, M.; Wang, X.; Ma, W.; Jiang, X.; Cui, J. Single-cell RNA sequencing highlights the functional role of human endogenous retroviruses in gallbladder cancer. EBioMedicine 2022, 85, 104319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Wu, X.-C.; Zhang, M.-D.; Weng, M.-Z.; Zhou, D.; Quan, Z.-W. Upregulation of H19 indicates a poor prognosis in gallbladder carcinoma and promotes epithelial-mesenchymal transition. Am. J. Cancer Res. 2016, 6, 15–26. [Google Scholar] [PubMed]
- Huang, Y.; Zhang, W.; Xu, C.; Li, Q.; Zhang, W.; Xu, W.; Zhang, M. Presence of PD-1 similarity genes in monocytes may promote the development of type 1 diabetes mellitus and poor prognosis of pancreatic cancer. BMJ Open Diabetes Res. Care 2023, 11, e003196. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Gardiner, J.C.; Maggi, E.C.; Huang, S.; Adem, A.; Bagdasarov, S.; Li, G.; Lee, S.; Slegowski, D.; Exarchakis, A.; et al. B7 immune-checkpoints as targets for the treatment of neuroendocrine tumors. Endocr. Relat. Cancer 2021, 28, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, J.; Liu, Y.; Zheng, X.; Feng, J.; Chen, X.; Jiang, T.; Li, Y.; Chen, L. Prognostic values of B7-H3, B7-H4, and HHLA2 expression in human pancreatic cancer tissues based on mIHC and spatial distribution analysis. Pathol. Res. Pract. 2022, 234, 153911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, H.; Yang, L.-L.; Mao, L.; Wu, C.-C.; Zhang, W.-F.; Sun, Z.-J. The Expression Patterns and Associated Clinical Parameters of Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 and Transmembrane and Immunoglobulin Domain Containing 2 in Oral Squamous Cell Carcinoma. Dis. Markers 2019, 2019, 5421985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, F.; Sun, N.; Zhang, Z.; Zhang, G.; Zhang, Z.; Luo, Y.; Che, Y.; Cheng, H.; Li, J.; et al. The combination of novel immune checkpoints HHLA2 and ICOSLG: A new system to predict survival and immune features in esophageal squamous cell carcinoma. Genes Dis. 2022, 9, 415–428. [Google Scholar] [CrossRef]
- Cheng, H.; Janakiram, M.; Borczuk, A.; Lin, J.; Qiu, W.; Liu, H.; Chinai, J.M.; Halmos, B.; Perez-Soler, R.; Zang, X. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status. Clin. Cancer Res. 2017, 23, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Jia, Q.; Chen, J.; Diao, X.; Gao, J.; Wang, X.; Zhu, B. Impaired Cytolytic Activity and Loss of Clonal Neoantigens in Elderly Patients With Lung Adenocarcinoma. J. Thorac. Oncol. 2019, 14, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cali Daylan, A.E.; Deng, L.; Yang, J.; Sharma, J.; Su, C.; Li, S.; Zang, X.; Halmos, B.; Borczuk, A.; et al. Heterogeneous Expression of PD-L1, B7x, B7-H3, and HHLA2 in Pulmonary Sarcomatoid Carcinoma and the Related Regulatory Signaling Pathways. Cancers 2023, 15, 3372. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhang, L.; Yang, Y.; Zhou, Y.; Chen, H. Different clinical significance of novel B7 family checkpoints VISTA and HHLA2 in human lung adenocarcinoma. Immunotherapy 2022, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Inal, C.; Yilmaz, E.; Piperdi, B.; Perez-Soler, R.; Cheng, H. Emerging treatment for advanced lung cancer with EGFR mutation. Expert Opin. Emerg. Drugs 2015, 20, 597–612. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Xie, Y.; Zhu, J.; Zhang, S.; Ge, Y.; Guo, J.; Luo, N.; Huang, W.; Xu, R.; et al. MUC16 stimulates neutrophils to an inflammatory and immunosuppressive phenotype in ovarian cancer. J. Ovarian Res. 2023, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, P.; Zheng, X.; Chen, L.; Kong, C.; Liu, W.; Li, S.; Jiang, J. Downregulation of HHLA2 inhibits ovarian cancer progression via the NF-κB signaling pathway and suppresses the expression of CA9. Cell. Immunol. 2023, 388–389, 104730. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Ouyang, D.; Qiao, Y.; Li, K.; Zheng, G.; Lao, X.; Zhang, S.; Liao, G.; Liang, Y. CA9 transcriptional expression determines prognosis and tumour grade in tongue squamous cell carcinoma patients. J. Cell. Mol. Med. 2020, 24, 5832–5841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccin. Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, J.; Zhu, M.; Wang, D.; Liu, H.; Zhan, P.; Yin, J.; Ye, M.; Song, Y.; Lv, T. The safety and efficacy of anti-PD-1 inhibitor-based combinational therapy in non-small cell lung cancer patients with oncogenic alterations. Transl. Cancer Res. 2024, 13, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cong, L.; Liu, J.; Peng, L.; Wang, J.; Feng, A.; Yue, J.; Li, L.; Wang, X.; Wang, X. The Efficacy and Safety of Regorafenib in Combination With Anti-PD-1 Antibody in Refractory Microsatellite Stable Metastatic Colorectal Cancer: A Retrospective Study. Front. Oncol. 2020, 10, 594125. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, A.; Koszewska, D.; Kot, A.; Dawidowicz, M.; Mielcarska, S.; Waniczek, D.; Świętochowska, E. The Importance of HHLA2 in Solid Tumors—A Review of the Literature. Cells 2024, 13, 794. https://doi.org/10.3390/cells13100794
Kula A, Koszewska D, Kot A, Dawidowicz M, Mielcarska S, Waniczek D, Świętochowska E. The Importance of HHLA2 in Solid Tumors—A Review of the Literature. Cells. 2024; 13(10):794. https://doi.org/10.3390/cells13100794
Chicago/Turabian StyleKula, Agnieszka, Dominika Koszewska, Anna Kot, Miriam Dawidowicz, Sylwia Mielcarska, Dariusz Waniczek, and Elżbieta Świętochowska. 2024. "The Importance of HHLA2 in Solid Tumors—A Review of the Literature" Cells 13, no. 10: 794. https://doi.org/10.3390/cells13100794





