Modification of Gas6 Protein in the Brain by a Functional Endogenous Tissue Vitamin K Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Mouse Glial Cell Cultures
2.2. Mouse Organotypic Cerebellar Slice Culture
2.3. Protein Extraction from Mouse Tissues and Isolation of Mouse Liver and Brain Microsomes
2.4. RT-qPCR
2.5. SDS-PAGE and Western Blotting
2.6. Immunoassays for Total and Carboxylated Gas6
2.7. Statistical Analysis
3. Results
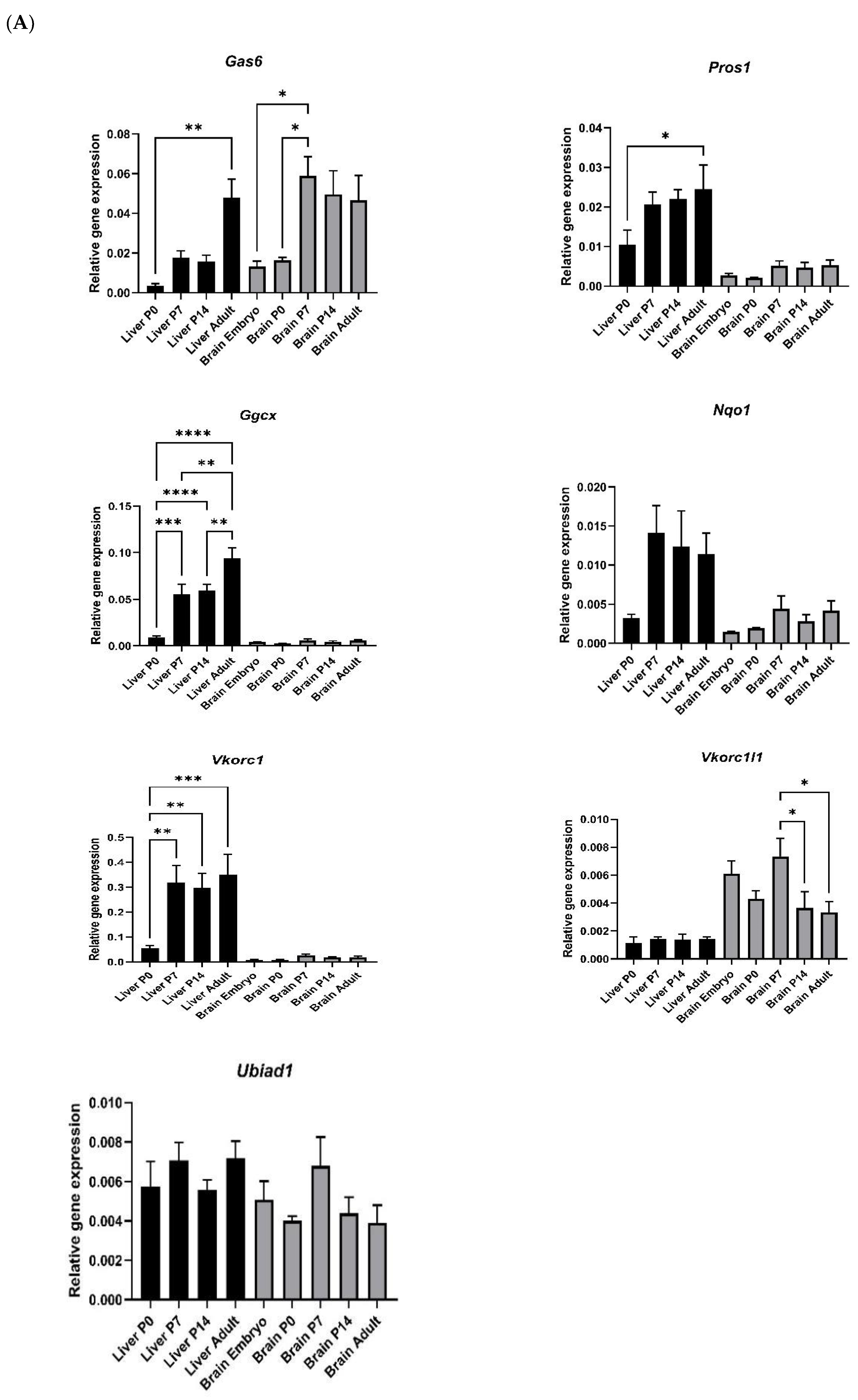
3.1. Gene Expression of TAM Receptors and TAM Ligands in Mouse Tissues and Brain Glial Cells
3.2. Expression of Vitamin K Cycle Enzyme Genes in the Mouse Brain during Embryonic and Postnatal Development and in Brain Glial Cells
3.3. Regulation of Expression of Vitamin K Cycle Enzyme and TAM Ligand Genes by Exogenous Agents in Cultured Mouse Brain Glial Cells
3.4. Warfarin Blocks the Functionality of the Vitamin K Cycle in Microglia and Astrocytes
3.5. Vitamin K Increases Gas6 γ-Carboxylation in Mouse Cerebellum
3.6. Warfarin Suppresses γ-Carboxylation of Gas6 in Rat Brain Tissue Ex Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hafizi, S.; Dahlback, B. Signalling and Functional Diversity within the Axl Subfamily of Receptor Tyrosine Kinases. Cytokine Growth Factor Rev. 2006, 17, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Dahlback, B. Gas6 and Protein S. Vitamin K-Dependent Ligands for the Axl Receptor Tyrosine Kinase Subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef]
- Nakano, T.; Higashino, K.; Kikuchi, N.; Kishino, J.; Nomura, K.; Fujita, H.; Ohara, O.; Arita, H. Vascular Smooth Muscle Cell-Derived, Gla-Containing Growth-Potentiating Factor for Ca(2+)-Mobilizing Growth Factors. J. Biol. Chem. 1995, 270, 5702–5705. [Google Scholar] [CrossRef] [PubMed]
- Goruppi, S.; Ruaro, E.; Schneider, C. Gas6, the Ligand of Axl Tyrosine Kinase Receptor, Has Mitogenic and Survival Activities for Serum Starved NIH3T3 Fibroblasts. Oncogene 1996, 12, 471–480. [Google Scholar] [PubMed]
- Angelillo-Scherrer, A.; Burnier, L.; Flores, N.; Savi, P.; DeMol, M.; Schaeffer, P.; Herbert, J.-M.; Lemke, G.; Goff, S.P.; Matsushima, G.K.; et al. Role of Gas6 Receptors in Platelet Signaling during Thrombus Stabilization and Implications for Antithrombotic Therapy. J. Clin. Investig. 2005, 115, 237–246. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Koller, B.H.; Earp, H.S.; Matsushima, G.K. A Novel Receptor Tyrosine Kinase, Mer, Inhibits TNF-Alpha Production and Lipopolysaccharide-Induced Endotoxic Shock. J. Immunol. 1999, 162, 3498–3503. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.L.; O’Guin, K.; Kim, M.; Varnum, B.; Lemke, G.; Brosnan, C.F.; Shafit-Zagardo, B. Gas6/Axl Signaling Activates the Phosphatidylinositol 3-Kinase/Akt1 Survival Pathway to Protect Oligodendrocytes from Tumor Necrosis Factorα-Induced Apoptosis. J. Neurosci. 2006, 26, 5638. [Google Scholar] [CrossRef]
- Yagami, T.; Ueda, K.; Asakura, K.; Okamura, N.; Sakaeda, T.; Sakaguchi, G.; Itoh, N.; Hashimoto, Y.; Nakano, T.; Fujimoto, M. Effect of Gas6 on Secretory Phospholipase A2-IIA-Induced Apoptosis in Cortical Neurons. Brain Res. 2003, 985, 142–149. [Google Scholar] [CrossRef]
- Binder, M.D.; Cate, H.S.; Prieto, A.L.; Kemper, D.; Butzkueven, H.; Gresle, M.M.; Cipriani, T.; Jokubaitis, V.G.; Carmeliet, P.; Kilpatrick, T.J. Gas6 Deficiency Increases Oligodendrocyte Loss and Microglial Activation in Response to Cuprizone-Induced Demyelination. J. Neurosci. 2008, 28, 5195–5206. [Google Scholar] [CrossRef] [PubMed]
- Tsiperson, V.; Li, X.; Schwartz, G.J.; Raine, C.S.; Shafit-Zagardo, B. GAS6 Enhances Repair Following Cuprizone-Induced Demyelination. PLoS ONE 2010, 5, e15748. [Google Scholar] [CrossRef]
- Berkner, K.L.; Runge, K.W. Vitamin K-Dependent Protein Activation: Normal Gamma-Glutamyl Carboxylation and Disruption in Disease. Int. J. Mol. Sci. 2022, 23, 5759. [Google Scholar] [CrossRef]
- Presnell, S.R.; Stafford, D.W. The Vitamin K-Dependent Carboxylase. Thromb. Haemost. 2002, 87, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Caspers, M.; Czogalla, K.J.; Liphardt, K.; Müller, J.; Westhofen, P.; Watzka, M.; Johannes, O. Two Enzymes Catalyze Vitamin K 2,3-Epoxide Reductase Activity in Mouse: VKORC1 Is Highly Expressed in Exocrine Tissues While VKORC1L1 Is Highly Expressed in Brain. Thromb Res. 2015, 135, 977–983. [Google Scholar] [CrossRef]
- Hammed, A.; Matagrin, B.; Spohn, G.; Prouillac, C.; Benoit, E.; Lattard, V. VKORC1L1, an Enzyme Rescuing the Vitamin K 2,3-Epoxide Reductase Activity in Some Extrahepatic Tissues during Anticoagulation Therapy. J. Biol. Chem. 2013, 288, 28733–28742. [Google Scholar] [CrossRef]
- Lacombe, J.; Rishavy, M.A.; Berkner, K.L.; Ferron, M. VKOR Paralog VKORC1L1 Supports Vitamin K-Dependent Protein Carboxylation in Vivo. JCI Insight. 2018, 3, e96501. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.-K.; Jin, D.-Y.; Straight, D.L.; Stafford, D.W. Functional Study of the Vitamin K Cycle in Mammalian Cells. Blood 2011, 117, 2967–2974. [Google Scholar] [CrossRef]
- Ingram, B.O.; Turbyfill, J.L.; Bledsoe, P.J.; Jaiswal, A.K.; Stafford, D.W. Assessment of the Contribution of NAD(P)H-Dependent Quinone Oxidoreductase 1 (NQO1) to the Reduction of Vitamin K in Wild-Type and NQO1-Deficient Mice. Biochem. J. 2013, 456, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gutala, R.; Jaiswal, A.K. Quinone Oxidoreductases and Vitamin K Metabolism. Vitam. Horm. 2008, 78, 85–101. [Google Scholar] [CrossRef]
- Aaseth, J.O.; Alehagen, U.; Opstad, T.B.; Alexander, J. Vitamin K and Calcium Chelation in Vascular Health. Biomedicines 2023, 11, 3154. [Google Scholar] [CrossRef]
- Oldenburg, J.; Marinova, M.; Müller-Reible, C.; Watzka, M. The Vitamin K Cycle. Vitam. Horm. 2008, 78, 35–62. [Google Scholar] [CrossRef]
- Geng, K. Post-Translational Modifications of the Ligands: Requirement for TAM Receptor Activation. Int. Rev. Cell Mol. Biol. 2020, 357, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, H.; Pienta, K.J. Axl as a Mediator of Cellular Growth and Survival. Oncotarget 2014, 5, 8818–8852. [Google Scholar] [CrossRef] [PubMed]
- Berkner, K.L.; Runge, K.W. The Physiology of Vitamin K Nutriture and Vitamin K-Dependent Protein Function in Atherosclerosis. J. Thromb. Haemost. 2004, 2, 2118–2132. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.F.; Hamulyák, K.; Knapen, M.H.J.; Vik, H.; Vermeer, C. Vitamin K-Containing Dietary Supplements: Comparison of Synthetic Vitamin K1 and Natto-Derived Menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef] [PubMed]
- Rishavy, M.A.; Hallgren, K.W.; Wilson, L.A.; Usubalieva, A.; Runge, K.W.; Berkner, K.L. The Vitamin K Oxidoreductase Is a Multimer That Efficiently Reduces Vitamin K Epoxide to Hydroquinone to Allow Vitamin K-Dependent Protein Carboxylation. J. Biol. Chem. 2013, 288, 31556. [Google Scholar] [CrossRef] [PubMed]
- Ferland, G. Vitamin K and Brain Function. Semin. Thromb. Hemost. 2013, 39, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of Phylloquinone (Vitamin K1) into Menaquinone-4 (Vitamin K2) in Mice: Two Possible Routes for Menaquinone-4 Accumulation in Cerebra of Mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Tsugawa, N.; Nakagawa, K.; Suhara, Y.; Tanaka, K.; Uchino, Y.; Takeuchi, A.; Sawada, N.; Kamao, M.; Wada, A.; et al. Menadione (Vitamin K 3 ) Is a Catabolic Product of Oral Phylloquinone (Vitamin K 1 ) in the Intestine and a Circulating Precursor of Tissue Menaquinone-4 (Vitamin K 2 ) in Rats *. J. Biol. Chem. 2013, 288, 33071–33080. [Google Scholar] [CrossRef] [PubMed]
- Presse, N.; Belleville, S.; Gaudreau, P.; Greenwood, C.E.; Kergoat, M.J.; Morais, J.A.; Payette, H.; Shatenstein, B.; Ferland, G. Vitamin K Status and Cognitive Function in Healthy Older Adults. Neurobiol. Aging 2013, 34, 2777–2783. [Google Scholar] [CrossRef]
- Chouet, J.; Ferland, G.; Féart, C.; Rolland, Y.; Presse, N.; Boucher, K.; Barberger-Gateau, P.; Beauchet, O.; Annweiler, C. Dietary Vitamin K Intake Is Associated with Cognition and Behaviour among Geriatric Patients: The CLIP Study. Nutrients 2015, 7, 6739–6750. [Google Scholar] [CrossRef]
- Booth, S.L.; Shea, M.K.; Barger, K.; Leurgans, S.E.; James, B.D.; Holland, T.M.; Agarwal, P.; Fu, X.; Wang, J.; Matuszek, G.; et al. Association of Vitamin K with Cognitive Decline and Neuropathology in Community-dwelling Older Persons. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12255. [Google Scholar] [CrossRef]
- Saputra, W.D.; Aoyama, N.; Komai, M.; Shirakawa, H. Menaquinone-4 Suppresses Lipopolysaccharide-Induced Inflammation in MG6 Mouse Microglia-Derived Cells by Inhibiting the NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 2317. [Google Scholar] [CrossRef]
- Mecha, M.; Iñigo, P.M.; Mestre, L.; Hernangómez, M.; Borrell, J.; Guaza, C. An Easy and Fast Way to Obtain a High Number of Glial Cells from Rat Cerebral Tissue: A Beginners Approach. Protoc. Exch. 2011. [Google Scholar] [CrossRef]
- Gilchrist, S.E.; Goudarzi, S.; Hafizi, S. Gas6 Inhibits Toll-Like Receptor-Mediated Inflammatory Pathways in Mouse Microglia via Axl and Mer. Front. Cell. Neurosci. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- De Simoni, A.; MY Yu, L. Preparation of Organotypic Hippocampal Slice Cultures: Interface Method. Nat. Protoc. 2006, 1, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Fannon, J.; Tarmier, W.; Fulton, D. Neuronal Activity and AMPA-Type Glutamate Receptor Activation Regulates the Morphological Development of Oligodendrocyte Precursor Cells. Glia 2015, 63, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Stoppini, L.; Buchs, P.A.; Muller, D. A Simple Method for Organotypic Cultures of Nervous Tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Swinny, J.D.; Metzger, F.; IJkema-Paassen, J.; Gounko, N.V.; Gramsbergen, A.; Van Der Want, J.J.L. Corticotropin-Releasing Factor and Urocortin Differentially Modulate Rat Purkinje Cell Dendritic Outgrowth and Differentiation in Vitro. Eur. J. Neurosci. 2004, 19, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, V.; Ananda Theertha Varada, H.K. High Activity of Cytochrome P-450-Linked Aminopyrine N-Demethylase in Mouse Brain Microsomes, and Associated Sex-Related Difference. Biochem. J. 1989, 261, 769. [Google Scholar] [CrossRef]
- Samanta, T.B.; Das, N.; Das, M.; Marik, R. Mechanism of Impairment of Cytochrome P450-Dependent Metabolism in Hamster Liver during Leishmaniasis. Biochem. Biophys. Res. Commun. 2003, 312, 75–79. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.A.; Rannels, S.R. Glucocorticoid Effects on Vitamin K-Dependent Carboxylase Activity and Matrix Gla Protein Expression in Rat Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L569–L577. [Google Scholar] [CrossRef] [PubMed]
- Tamadon-Nejad, S.; Ouliass, B.; Rochford, J.; Ferland, G. Vitamin K Deficiency Induced by Warfarin Is Associated With Cognitive and Behavioral Perturbations, and Alterations in Brain Sphingolipids in Rats. Front. Aging Neurosci. 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, S.; Rivera, A.; Butt, A.M.; Hafizi, S. Gas6 Promotes Oligodendrogenesis and Myelination in the Adult Central Nervous System and After Lysolecithin-Induced Demyelination. ASN Neuro 2016, 8, 1759091416668430. [Google Scholar] [CrossRef]
- Prieto, A.L.; Weber, J.L.; Tracy, S.; Heeb, M.J.; Lai, C. Gas6, a Ligand for the Receptor Protein-Tyrosine Kinase Tyro-3, Is Widely Expressed in the Central Nervous System. Brain Res. 1999, 816, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Totoki, T.; D’Alessandro-Gabazza, C.N.; Toda, M.; Tonto, P.B.; Takeshita, A.; Yasuma, T.; Nishihama, K.; Iwasa, M.; Horiki, N.; Takei, Y.; et al. Protein S Exacerbates Chronic Liver Injury and Fibrosis. Am. J. Pathol. 2018, 188, 1195–1203. [Google Scholar] [CrossRef]
- Fourgeaud, L.; Traves, P.G.; Tufail, Y.; Leal-Bailey, H.; Lew, E.D.; Burrola, P.G.; Callaway, P.; Zagorska, A.; Rothlin, C.V.; Nimmerjahn, A.; et al. TAM Receptors Regulate Multiple Features of Microglial Physiology. Nature 2016, 532, 240–244. [Google Scholar] [CrossRef]
- Ji, R.; Tian, S.; Lu, H.J.; Lu, Q.; Zheng, Y.; Wang, X.; Ding, J.; Li, Q.; Lu, Q. TAM Receptors Affect Adult Brain Neurogenesis by Negative Regulation of Microglial Cell Activation. J. Immunol. 2013, 191, 6165–6177. [Google Scholar] [CrossRef]
- Moreno, P.; Fexova, S.; George, N.; Manning, J.R.; Miao, Z.; Mohammed, S.; Muñoz-Pomer, A.; Fullgrabe, A.; Bi, Y.; Bush, N.; et al. Expression Atlas Update: Gene and Protein Expression in Multiple Species. Nucleic Acids Res. 2022, 50, D129–D140. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34, 11929. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Lemke, G. An Extended Family of Protein-Tyrosine Kinase Genes Differentially Expressed in the Vertebrate Nervous System. Neuron 1991, 6, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. How Macrophages Deal with Death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.S.; McMahon, E.J.; Pop, S.M.; Reap, E.A.; Caricchio, R.; Cohen, P.L.; Shelton Earp, H.; Matsushima, G.K. Phagocytosis and Clearance of Apoptotic Cells Is Mediated by MER. Nature 2001, 411, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Través, P.G.; Lew, E.D.; Dransfield, I.; Lemke, G. Diversification of TAM Receptor Function. Nat. Immunol. 2014, 15, 920–928. [Google Scholar] [CrossRef]
- Hubbard, B.R.; Ulrich, M.M.W.; Jacobs, M.; Vermeer, C.; Walsh, C.; Furie, B.; Furie, B.C. Vitamin K-Dependent Carboxylase: Affinity Purification from Bovine Liver by Using a Synthetic Propeptide Containing the Gamma-Carboxylation Recognition Site. Proc. Natl. Acad. Sci. USA 1989, 86, 6893. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.L.; Weber, J.L.; Lai, C. Expression of the Receptor Protein-Tyrosine Kinases Tyro-3, Axl, and Mer in the Developing Rat Central Nervous System. J. Comp. Neurol. 2000, 425, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Ferron, M. VKORC1L1, An Enzyme Mediating the Effect of Vitamin K in Liver and Extrahepatic Tissues. Nutrients 2018, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Margarida Cardoso Moreira Mouse RNA-Seq Time-Series of the Development of Seven Major Organs. Available online: https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-6798 (accessed on 18 March 2024).
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A Non-Canonical Vitamin K Cycle Is a Potent Ferroptosis Suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sawada, N.; Hirota, Y.; Uchino, Y.; Suhara, Y.; Hasegawa, T.; Amizuka, N.; Okamoto, T.; Tsugawa, N.; Kamao, M.; et al. Vitamin K2 Biosynthetic Enzyme, UBIAD1 Is Essential for Embryonic Development of Mice. PLoS ONE 2014, 9, e104078. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a Novel Human Menaquinone-4 Biosynthetic Enzyme. Nature 2010, 468, 117. [Google Scholar] [CrossRef] [PubMed]
- Carrié, I.; Portoukalian, J.; Vicaretti, R.; Rochford, J.; Potvin, S.; Ferland, G. Menaquinone-4 Concentration Is Correlated with Sphingolipid Concentrations in Rat Brain. J. Nutr. 2004, 134, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-Inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, N.; Ouliass, B.; Ferland, G.; Hafizi, S. Modification of Gas6 Protein in the Brain by a Functional Endogenous Tissue Vitamin K Cycle. Cells 2024, 13, 873. https://doi.org/10.3390/cells13100873
Aydin N, Ouliass B, Ferland G, Hafizi S. Modification of Gas6 Protein in the Brain by a Functional Endogenous Tissue Vitamin K Cycle. Cells. 2024; 13(10):873. https://doi.org/10.3390/cells13100873
Chicago/Turabian StyleAydin, Nadide, Bouchra Ouliass, Guylaine Ferland, and Sassan Hafizi. 2024. "Modification of Gas6 Protein in the Brain by a Functional Endogenous Tissue Vitamin K Cycle" Cells 13, no. 10: 873. https://doi.org/10.3390/cells13100873




