Towards a UK Airborne Bioaerosol Climatology: Real-Time Monitoring Strategies for High Time Resolution Bioaerosol Classification and Quantification
Abstract
:1. Introduction
1.1. Detection Methods
1.2. Aims and Objectives
- Demonstrate the performance of a new dimensional reduction-based real-time BioPM classifier.
- Deploy the real-time system to characterize and quantify seasonal BioPM at two UK sites of interest.
- Investigate the influence of environmental factors on BioPM emission.
2. Methods
2.1. The Multiparameter Bioaerosol Spectrometer
- Tyrosine: 310 ± 20 nm;
- Tryptophan: 365 ± 40 nm;
- Riboflavin: 520 ± 30 nm;
- Chlorophyll b: 640 ± 10 nm.
2.2. Site Descriptions
2.2.1. Cardington
2.2.2. Weybourne Atmospheric Observatory
- MBS, 15 September 2020 to 3 November 2020 (50 days);
- MBS, 15 April 2021 to 16 July 2020 (93 days).
2.3. Classification Method
- Characterizing the autofluorescent emission of each test species over 8 narrow bands between 315 and 640 nm after deep UV excitation at 280 nm. This probes the relative biofluorophore makeup of the bioaerosol under test.
- Characterizing particle morphology via interrogating two chords of the 2D scattering image with a dual CMOS detector. This delivers proxy information on morphological features such as particle sphericity/aspect ratio and surface roughness. Additionally, particle size is determined via Mie scattering.
3. Results and Discussion
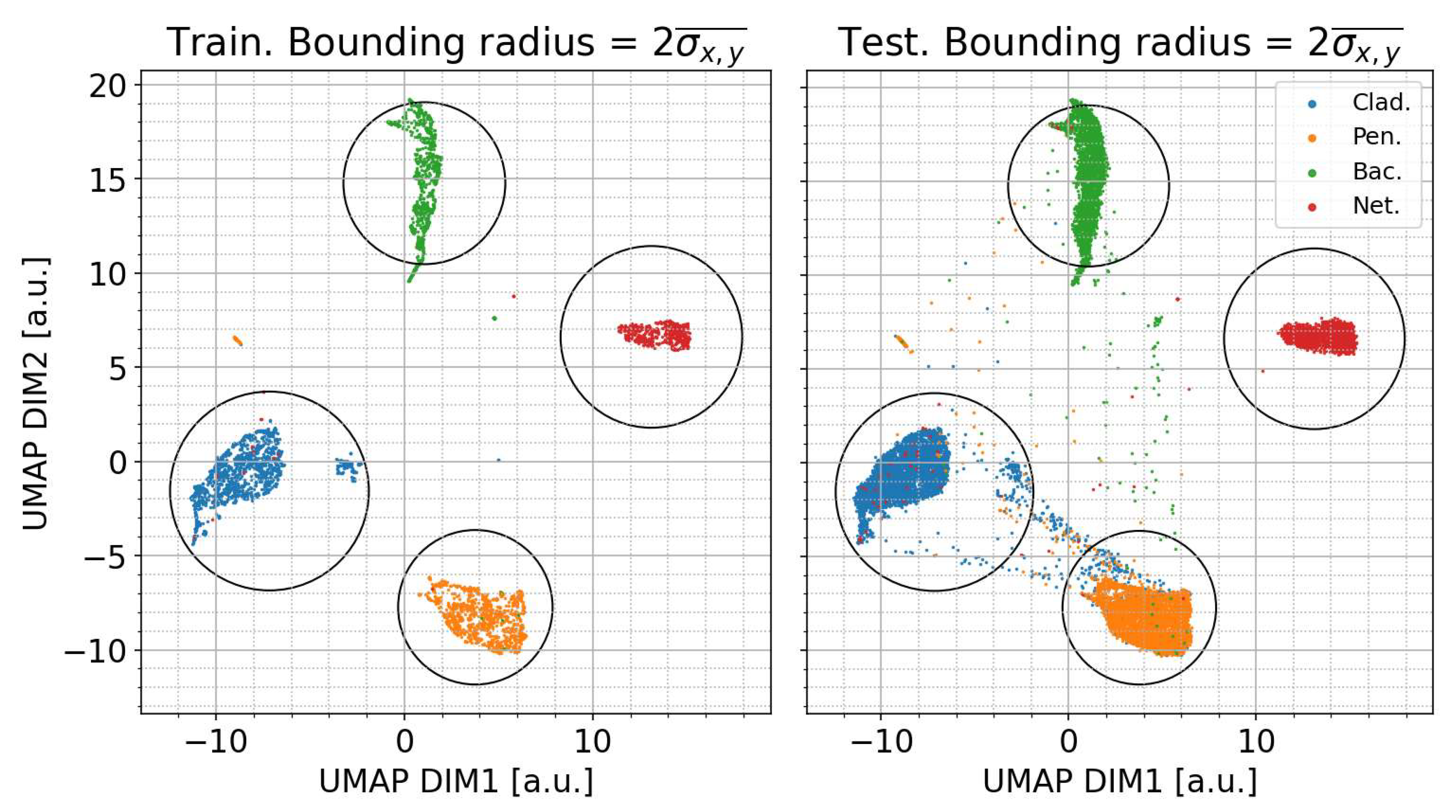
3.1. UMAP Classifier Training and Performance
3.2. Cardington 2019
3.3. Weybourne 2020 and 2021
3.4. Site Synthesis and Relationships
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BioPM | Biological particulate matter |
| ChAMBRe | Chamber for Aerosol Modeling and Bio-aerosol Research |
| CMOS | Complementary metal-oxide semiconductor |
| FT | Forced trigger |
| LPM | Litres per minute |
| MBS | Multiparameter bioaerosol spectrometer |
| MIDAS | Met Office Integrated Data Archive System |
| UMAP | Uniform manifold approximation and projection for dimension reduction |
| UV-LIF | Ultraviolet light-induced fluorescence |
| WIBS | Wideband integrated bioaerosol spectrometer |
References
- Bauer, H.; Kasper-Giebl, A.; Löflund, M.; Giebl, H.; Hitzenberger, R.; Zibuschka, F.; Puxbaum, H. The Contribution of Bacteria and Fungal Spores to the Organic Carbon Content of Cloud Water, Precipitation and Aerosols. Atmos. Res. 2002, 64, 109–119. [Google Scholar] [CrossRef]
- Bauer, H.; Schueller, E.; Weinke, G.; Berger, A.; Hitzenberger, R.; Marr, I.L.; Puxbaum, H. Significant Contributions of Fungal Spores to the Organic Carbon and to the Aerosol Mass Balance of the Urban Atmospheric Aerosol. Atmos. Environ. 2008, 42, 5542–5549. [Google Scholar] [CrossRef]
- Burrows, S.M.; Butler, T.; Jöckel, P.; Tost, H.; Kerkweg, A.; Pöschl, U.; Lawrence, M.G. Bacteria in the Global Atmosphere-Part 2: Modeling of Emissions and Transport between Different Ecosystems. Atmos. Chem. Phys. 2009, 9, 9281–9297. [Google Scholar] [CrossRef]
- Coulon, F.; Colbeck, I. Rambie, Rapid Monitoring of Bioaerosols in Urban, Agricultural and Industrial Environments, Nerc. Impact 2017, 2017, 12–14. [Google Scholar] [CrossRef]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field Sampling of Indoor Bioaerosols. Aerosol Sci. Technol. 2020, 54, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Crawford, I.; Gallagher, M.W.; Bower, K.N.; Choularton, T.W.; Flynn, M.J.; Ruske, S.; Listowski, C.; Brough, N.; Lachlan-Cope, T.; Zoë, L.; et al. Real-Time Detection of Airborne Fluorescent Bioparticles in Antarctica. Atmos. Chem. Phys. 2017, 17, 14291–14307. [Google Scholar] [CrossRef]
- Firacative, C. Invasive Fungal Disease in Humans: Are We Aware of the Real Impact? Mem. Inst. Oswaldo Cruz 2020, 115, e200430. [Google Scholar] [CrossRef]
- Iacobucci, G. Asthma Deaths Rise 33% in Past Decade in England and Wales. BMJ 2019, 366, l5108. [Google Scholar] [CrossRef]
- Sadyś, M.; Adams-Groom, B.; Herbert, R.J.; Kennedy, R. Comparisons of Fungal Spore Distributions Using Air Sampling at Worcester, England (2006–2010). Aerobiologia 2016, 32, 619–634. [Google Scholar] [CrossRef]
- Khot, A.; Burn, R. Seasonal Variation and Time Trends of Deaths from Asthma in England and Wales 1960-82. Br. Med. J. (Clin. Res. Ed.) 1984, 289, 233–234. [Google Scholar] [CrossRef]
- Sedghy, F.; Varasteh, A.R.; Sankian, M.; Moghadam, M. Interaction between Air Pollutants and Pollen Grains: The Role on the Rising Trend in Allergy. Rep. Biochem. Mol. Biol. 2018, 6, 219–224. [Google Scholar] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Cheng, T.L.; Reichard, J.D.; Coleman, J.T.H.; Weller, T.J.; Thogmartin, W.E.; Reichert, B.E.; Bennett, A.B.; Broders, H.G.; Campbell, J.; Etchison, K.; et al. The Scope and Severity of White-Nose Syndrome on Hibernating Bats in North America. Conserv. Biol. 2021, 35, 1586–1597. [Google Scholar] [CrossRef]
- Vali, G. Ice Nucleation-Review; Kulmala, M., Wagner, P., Eds.; Pergamon Press: Kidlington, UK, 1996; pp. 271–279. [Google Scholar]
- Knopf, D.A.; Alpert, P.A.; Wang, B. The Role of Organic Aerosol in Atmospheric Ice Nucleation: A Review. ACS Earth Space Chem. 2018, 2, 168–202. [Google Scholar] [CrossRef]
- Kunert, A.T.; Pöhlker, M.L.; Tang, K.; Krevert, C.S.; Wieder, C.; Speth, K.R.; Hanson, L.E.; Morris, C.E.; Schmale III, D.G.; Pöschl, U.; et al. Macromolecular Fungal Ice Nuclei in Fusarium: Effects of Physical and Chemical Processing. Biogeosciences 2019, 16, 4647–4659. [Google Scholar] [CrossRef]
- Amato, P.; Joly, M.; Besaury, L.; Oudart, A.; Taib, N.; Mone, A.I.; Deguillaume, L.; Delort, A.M.; Debroas, D. Active Microorganisms Thrive among Extremely Diverse Communities in Cloud Water. PLoS ONE 2017, 12, e0182869. [Google Scholar] [CrossRef]
- Song, H.; Crawford, I.; Lloyd, J.; Robinson, C.; Boothman, C.; Bower, K.; Gallagher, M.; Allen, G.; Topping, D. Airborne Bacterial and Eukaryotic Community Structure across the United Kingdom Revealed by High-Throughput Sequencing. Atmosphere 2020, 11, 802. [Google Scholar] [CrossRef]
- Twohy, C.H.; McMeeking, G.R.; DeMott, P.J.; McCluskey, C.S.; Hill, T.C.J.; Burrows, S.M.; Kulkarni, G.R.; Tanarhte, M.; Kafle, D.N.; Toohey, D.W.; et al. Abundance of Fluorescent Biological Aerosol Particles at Temperatures Conducive to the Formation of Mixed-Phase and Cirrus Clouds. Atmos. Chem. Phys. 2016, 16, 8205–8225. [Google Scholar] [CrossRef]
- Crawford, I.; Bower, K.N.; Choularton, T.W.; Dearden, C.; Crosier, J.; Westbrook, C.; Capes, G.; Coe, H.; Connolly, P.J.; Dorsey, J.R.; et al. Ice Formation and Development in Aged, Wintertime Cumulus over the Uk: Observations and Modelling. Atmos. Chem. Phys. 2012, 12, 4963–4985. [Google Scholar] [CrossRef]
- Huffman, J.A.; Prenni, A.J.; DeMott, P.J.; Pöhlker, C.; Mason, R.H.; Robinson, N.H.; Fröhlich-Nowoisky, J.; Tobo, Y.; Després, V.R.; Garcia, E.; et al. High Concentrations of Biological Aerosol Particles and Ice Nuclei During and after Rain. Atmos. Chem. Phys. 2013, 13, 6151–6164. [Google Scholar] [CrossRef]
- Niu, M.; Hu, W.; Cheng, B.; Wu, L.; Ren, L.; Deng, J.; Shen, F.; Fu, P. Influence of Rainfall on Fungal Aerobiota in the Urban Atmosphere over Tianjin, China: A Case Study. Atmos. Environ. X. 2021, 12, 100137. [Google Scholar] [CrossRef]
- Morris, C.E.; Conen, F.; Huffman, J.A.; Phillips, V.; Pöschl, U.; Sands, D.C. Bioprecipitation: A Feedback Cycle Linking Earth History, Ecosystem Dynamics and Land Use through Biological Ice Nucleators in the Atmosphere. Glob. Chang. Biol. 2014, 20, 341–351. [Google Scholar] [CrossRef]
- Sands, D.C.; Langhans, V.E.; Scharen, A.L.; de Smet, G. The Association between Bacteria and Rain and Possible Resultant Meteorological Implications. J. Hung. Meteorol. Serv. 1982, 86, 148–152. [Google Scholar]
- Huffman, J.A.; Perring, A.E.; Savage, N.J.; Clot, B.; Crouzy, B.; Tummon, F.; Shoshanim, O.; Damit, B.; Schneider, J.; Sivaprakasam, V.; et al. Real-Time Sensing of Bioaerosols: Review and Current Perspectives. Aerosol Sci. Technol. 2019, 54, 465–495. [Google Scholar] [CrossRef]
- Whitby, C.; Ferguson, R.M.W.; Colbeck, I.; Dumbrell, A.J.; Nasir, Z.A.; Marczylo, E.; Kinnersley, R.; Douglas, P.; Drew, G.; Bhui, K.; et al. Compendium of Analytical Methods for Sampling, Characterization and Quantification of Bioaerosols. Funct. Microbiomes 2022, 67, 101–229. [Google Scholar]
- Pasquarella, C.; Albertini, R.; Dall, P.; Saccani, E.; Sansebastiano, G.E.; Signorelli, C. Air Microbial Sampling: The State of the Art. Ig. Sanità Pubblica 2008, 64, 79–120. [Google Scholar]
- Viani, I.; Colucci, M.E.; Pergreffi, M.; Rossi, D.; Veronesi, L.; Bizzarro, A.; Capobianco, E.; Affanni, P.; Zoni, R.; Saccani, E.; et al. Passive Air Sampling: The Use of the Index of Microbial Air Contamination. Acta Biomed. 2020, 91, 92–105. [Google Scholar] [CrossRef]
- Crawford, I.; Topping, D.; Gallagher, M.; Forde, E.; Lloyd, J.R.; Foot, V.; Stopford, C.; Kaye, P. Detection of Airborne Biological Particles in Indoor Air Using a Real-Time Advanced Morphological Parameter Uv-Lif Spectrometer and Gradient Boosting Ensemble Decision Tree Classifiers. Atmosphere 2020, 11, 1039. [Google Scholar] [CrossRef]
- Ruske, S.; Topping, D.O.; Foot, V.E.; Kaye, P.H.; Stanley, W.R.; Crawford, I.; Morse, A.P.; Gallagher, M.W. Evaluation of Machine Learning Algorithms for Classification of Primary Biological Aerosol Using a New Uv-Lif Spectrometer. Atmos. Meas. Tech. 2017, 10, 695–708. [Google Scholar] [CrossRef]
- Forde, E.; Gallagher, M.; Walker, M.; Foot, V.; Attwood, A.; Granger, G.; Sarda-Estève, R.; Stanley, W.; Kaye, P.; Topping, D. Intercomparison of Multiple Uv-Lif Spectrometers Using the Aerosol Challenge Simulator. Atmosphere 2019, 10, 797. [Google Scholar] [CrossRef]
- Könemann, T.; Savage, N.; Klimach, T.; Walter, D.; Fröhlich-Nowoisky, J.; Su, H.; Pöschl, U.; Huffman, J.A.; Pöhlker, C. Spectral Intensity Bioaerosol Sensor (Sibs): An Instrument for Spectrally Resolved Fluorescence Detection of Single Particles in Real Time. Atmos. Meas. Tech. 2019, 12, 1337–1363. [Google Scholar] [CrossRef]
- Šaulienė, .; Šukienė, L.; Daunys, G.; Valiulis, G.; Vaitkevičius, L.; Matavulj, P.; Brdar, S.; Panic, M.; Sikoparija, B.; Clot, B.; et al. Automatic Pollen Recognition with the Rapid-E Particle Counter: The First-Level Procedure, Experience and Next Steps. Atmos. Meas. Tech. 2019, 12, 3435–3452. [Google Scholar] [CrossRef]
- Freitas, G.P.; Stolle, C.; Kaye, P.H.; Stanley, W.; Herlemann, D.P.R.; Salter, M.E.; Zieger, P. Emission of Primary Bioaerosol Particles from Baltic Seawater. Environ. Sci. Atmos. 2022, 2, 1170–1182. [Google Scholar] [CrossRef]
- Savage, N.; Krentz, C.; Könemann, T.; Han, T.T.; Mainelis, G.; Pöhlker, C.; Huffman, J.A. Systematic Characterization and Fluorescence Threshold Strategies for the Wideband Integrated Bioaerosol Sensor (Wibs) Using Size-Resolved Biological and Interfering Particles. Atmos. Meas. Tech. Discuss. 2017, 10, 4279–4302. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J.; Melville, J. Umap: Uniform Manifold Approximation and Projection for Dimension Reduction. Arxiv Mach. Learn. 2018, 3, 861. [Google Scholar] [CrossRef]
- Ruske, S.; Topping, D.O.; Foot, V.E.; Morse, A.P.; Gallagher, M.W. Machine Learning for Improved Data Analysis of Biological Aerosol Using the Wibs. Atmos. Meas. Tech. 2018, 11, 6203–6230. [Google Scholar] [CrossRef]
- Danelli, S.G.; Brunoldi, M.; Massabò, D.; Parodi, F.; Vernocchi, V.; Prati, P. Comparative Characterization of the Performance of Bio-Aerosol Nebulizers in Connection with Atmospheric Simulation Chambers. Atmos. Meas. Tech. 2021, 14, 4461–4470. [Google Scholar] [CrossRef]
- Massabò, D.; Danelli, S.G.; Brotto, P.; Comite, A.; Costa, C.; Di Cesare, A.; Doussin, J.F.; Ferraro, F.; Formenti, P.; Gatta, E.; et al. Chambre: A New Atmospheric Simulation Chamber for Aerosol Modelling and Bio-Aerosol Research. Atmos. Meas. Tech. 2018, 11, 5885–5900. [Google Scholar] [CrossRef]
- Hirst, J.M. Changes in Atmospheric Spore Content: Diurnal Periodicity and the Effects of Weather. Trans. Br. Mycol. Soc. 1953, 36, 375–393. [Google Scholar] [CrossRef]
- Oneto, D.L.; Golan, J.; Mazzino, A.; Pringle, A.; Seminara, A. Timing of Fungal Spore Release Dictates Survival during Atmospheric Transport. Proc. Natl. Acad. Sci. USA 2020, 117, 5134–5143. [Google Scholar] [CrossRef]
- MetOffice. Met Office Integrated Data Archive System (Midas) Land and Marine Surface Stations Data (1853-Current); NCAS British Atmospheric Data Centre: Leeds, UK, 2012. [Google Scholar]
- Maki, T.K.H.; Hosaka, K.; Lee, K.C.; Kawabata, Y.; Kajino, M.; Uto, M.; Kita, K.; Igarashi, Y. Vertical Distribution of Airborne Microorganisms over Forest Environments: A Potential Source of Ice-Nucleating Bioaerosols. Atmos. Environ. 2023, 302, 119726. [Google Scholar] [CrossRef]
- Heo, K.J.; Jeong, S.B.; Lim, C.E.; Lee, G.W.; Lee, B.U. Diurnal Variation in Concentration of Culturable Bacterial and Fungal Bioaerosols in Winter to Spring Season. Atmosphere 2023, 14, 537. [Google Scholar] [CrossRef]
- Whitehead, J.D.; Darbyshire, E.; Brito, J.; Barbosa, H.M.J.; Crawford, I.; Stern, R.; Gallagher, M.W.; Kaye, P.H.; Allan, J.D.; Coe, H.; et al. Biogenic Cloud Nuclei in the Central Amazon during the Transition from Wet to Dry Season. Atmos. Chem. Phys. 2016, 16, 9727–9743. [Google Scholar] [CrossRef]
- Gosselin, M.I.; Rathnayake, C.M.; Crawford, I.; Pöhlker, C.; Fröhlich-Nowoisky, J.; Schmer, B.; Després, V.R.; Engling, G.; Gallagher, M.; Stone, E.; et al. Fluorescent Bioaerosol Particle, Molecular Tracer, and Fungal Spore Concentrations during Dry and Rainy Periods in a Semi-Arid Forest. Atmos. Chem. Phys. 2016, 16, 15165–15184. [Google Scholar] [CrossRef]
- Crawford, I.; Robinson, N.H.; Flynn, M.J.; Foot, V.E.; Gallagher, M.W.; Huffman, J.A.; Stanley, W.R.; Kaye, P.H. Characterisation of Bioaerosol Emissions from a Colorado Pine Forest: Results from the Beachon-Rombas Experiment. Atmos. Chem. Phys. 2014, 14, 8559–8578. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, I.; Bower, K.; Topping, D.; Di Piazza, S.; Massabò, D.; Vernocchi, V.; Gallagher, M. Towards a UK Airborne Bioaerosol Climatology: Real-Time Monitoring Strategies for High Time Resolution Bioaerosol Classification and Quantification. Atmosphere 2023, 14, 1214. https://doi.org/10.3390/atmos14081214
Crawford I, Bower K, Topping D, Di Piazza S, Massabò D, Vernocchi V, Gallagher M. Towards a UK Airborne Bioaerosol Climatology: Real-Time Monitoring Strategies for High Time Resolution Bioaerosol Classification and Quantification. Atmosphere. 2023; 14(8):1214. https://doi.org/10.3390/atmos14081214
Chicago/Turabian StyleCrawford, Ian, Keith Bower, David Topping, Simone Di Piazza, Dario Massabò, Virginia Vernocchi, and Martin Gallagher. 2023. "Towards a UK Airborne Bioaerosol Climatology: Real-Time Monitoring Strategies for High Time Resolution Bioaerosol Classification and Quantification" Atmosphere 14, no. 8: 1214. https://doi.org/10.3390/atmos14081214






