Research on the Reconstruction of Aquatic Vegetation Landscape in Coal Mining Subsidence Wetlands Based on Ecological Water Level
Abstract
:1. Introduction
2. Materials and Methods
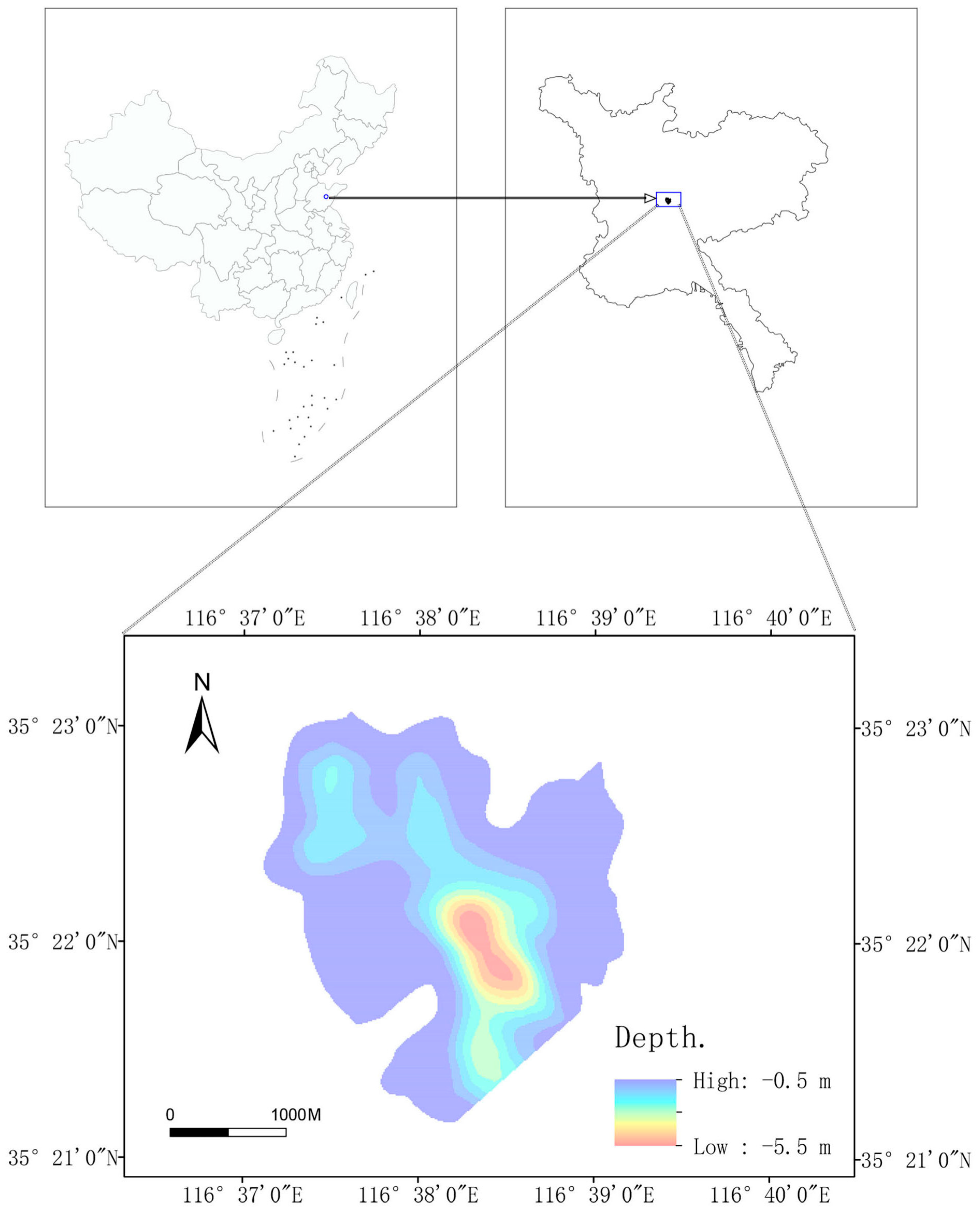
2.1. Research Area
2.2. Data Materials
2.3. Research Methods
2.3.1. Lake Morphology Analysis Method
2.3.2. Biological Space Minimum Demand Method
2.3.3. Water Level Demand Method for Aquatic Plants
3. Results
3.1. Species of Aquatic Plants
3.2. The Minimum Ecological Water Level
3.3. The Reconstruction Area and Coverage of Aquatic Vegetation
3.4. Recommended Monthly Water Levels for Various Stages of Aquatic Vegetation Growth
4. Discussion
4.1. Water Level Fluctuations and the Reconstruction of Coal Mining Subsidence Wetland Aquatic Biota
4.2. Water Level Fluctuations and the Dynamic Feedback Mechanism of Aquatic Vegetation Landscape Reconstruction throughout the Lifecycle of Various Coal Mining Subsidence Wetlands
4.3. Water Level Fluctuations and Carbon Sequestration of Aquatic Vegetation in Coal Mining Subsidence Wetlands
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, F. The influence and comprehensive improvement of the surface subsidence on the environment. Environ. Sci. 1988, 7, 72–75. (In Chinese) [Google Scholar] [CrossRef]
- Bian, Z.; Xue, W.; Wang, Q.; Lu, N.; Fan, H. Preliminary study on the dynamic process of goaf subsidence wetland formation. Wetl. Sci. 2007, 2, 124–127. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, C.; Wang, Q.; Liu, H.; Li, D.; Zhu, Q.; Liu, F. Seasonal characteristics of groundwater discharge controlled by precipitation and its environmental effects in a coal mining subsidence lake, eastern China. Sci. Total Environ. 2024, 915, 170067. [Google Scholar] [CrossRef]
- Zhou, S. Study on the Evaluation and Forewarning of Wetland Landscape Ecological Security for the Col Resource-Based Cities Based on Scenario Simulation. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2020. [Google Scholar]
- Wang, Z.; Zhang, Q.; Li, R. Study on rain-flood resources comprehensive utilization and ecological restoration technology of coal mining depressed area. J. Nat. Resour. 2009, 24, 1155–1162. [Google Scholar]
- Yuan, X.; Zhang, C.; Zhang, G.; Huang, X. Ecological Restoration Model of Coal Mining Subsidence Area Based on Biodiversity Conservation. Landsc. Archit. 2022, 29, 52–57. [Google Scholar]
- Hosper, S. Biomanipulation, new perspectives for restoration of shallow, eutrophic lakes in The Netherlands. Hydrobiol. Bull. 1989, 23, 5–10. [Google Scholar] [CrossRef]
- Whigham, D.F.; Simpson, R.L. The relationship between aboveground and belowground biomass of freshwater tidal wetland macrophytes. Aquat. Bot. 1978, 5, 355–364. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, L.; Zhou, L.; Gu, C.; Zhu, W. Bird diversity in summer in Anqing floodplain wetlands, middle-lower reaches of the Yangtze River. J. Lake Sci 2013, 25, 872–882. [Google Scholar]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Sun, Z.; Jiang, C. Study on ecological water level of Xuanwu Lake based on aquatic plant restoration. Adv. Sci. Technol. Water Resour. 2023, 43, 49–54+76. (In Chinese) [Google Scholar]
- Cuenca, M.C.; Hooper, A.J.; Hanssen, R.F. Surface deformation induced by water influx in the abandoned coal mines in Limburg, The Netherlands observed by satellite radar interferometry. J. Appl. Geophys. 2013, 88, 1–11. [Google Scholar] [CrossRef]
- Safronova, O.; Lamanova, T.; Sheremet, N.; Doronkin, V. Species composition dynamics in successive plant assemblages on the northern slopes of the coal mining spoils in the arid areas of Khakassia. BIO Web Conf. 2018, 11, 00036. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Guo, Y.; Li, D.; Yang, D.; Lu, Z. The relationship between understory plant diversity and soil factors in coal mining subsidence areas. J. Soil Water Conserv. 2022, 36, 268–276. (In Chinese) [Google Scholar]
- Zapico, I.; Duque, J.F.M.; Bugosh, N.; Laronne, J.B.; Ortega, A.; Molina, A.; Martín-Moreno, C.; Nicolau, J.M.; Castillo, L.S. Geomorphic reclamation for reestablishment of landform stability at a watershed scale in mined sites: The Alto Tajo Natural Park, Spain. Ecol. Eng. 2018, 111, 100–116. [Google Scholar] [CrossRef]
- Robinson, M. Changes in catchment runoff following drainage and afforestation. J. Hydrol. 1986, 86, 71–84. [Google Scholar] [CrossRef]
- Bai, J.; Ouyang, H.; Cui, B.; Wang, Q.; Chen, H. Changes in landscape pattern of alpine wetlands on the Zoige Plateau in the past four decades. Acta Ecol. Sin. 2008, 28, 2245–2252. [Google Scholar]
- Ertsen, M.W.; van der Spek, J. Modeling an irrigation ditch opens up the world. Hydrology and hydraulics of an ancient irrigation system in Peru. Phys. Chem. Earth Parts A/B/C 2009, 34, 176–191. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Feng, X.; Chen, X.; Yao, K. Evolution of stream-aquifer hydrologic connectedness during pumping–experiment. J. Hydrol. 2011, 402, 401–414. [Google Scholar] [CrossRef]
- Shore, M.; Mechan, S.; Cushen, M.; Jordan, P.; Mellander, P.; Kelly-Quinn, M.; Melland, A. Extent and role of ditches in affecting hydrological connectivity in agricultural landscapes. In Proceedings of the EGU General Assembly Conference, Vienna, Austria, 22–27 April 2012; pp. 22–27. [Google Scholar]
- Shabanov, N.; Vargas, M.; Miura, T.; Sei, A.; Danial, A. Evaluation of the performance of Suomi NPP VIIRS top of canopy vegetation indices over AERONET sites. Remote Sens. Environ. 2015, 162, 29–44. [Google Scholar] [CrossRef]
- Qiu, M.; Han, M.; Jiao, C.; Song, S.; Liu, Y. Estimation of Ecological Water Demand in the Yellow River Estuary Wetland. J. Ecol. 2023, 43, 9096–9105. (In Chinese) [Google Scholar]
- Xu, Z.; Chen, M.; Dong, Z. Researches on the calculation methods of the lowest ecological water level of lake. Acta Ecol. Sin. 2004, 24, 2324–2328. [Google Scholar]
- Li, X.; Song, Y.; Li, Y.; Xing, X.; Zhang, S. Calculation methods of lowest ecological water level of lake. Arid Land Geogr. 2007, 30, 526–530. [Google Scholar]
- Yang, W.; Xu, M.; Li, R.; Zhang, L.; Deng, Q. Estimating the ecological water levels of shallow lakes: A case study in Tangxun Lake, China. Sci. Rep. 2020, 10, 5637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cha, L. Theoretical exploration on the reclamation model of coal mining subsidence land. Energy Environ. Prot. 2008, 22, 1–4. (In Chinese) [Google Scholar]
- Wei, T. Effects on Runoff Caused by Coal Mining Subsidence Based on SWAT Model in Sihe Basin. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2015. [Google Scholar]
- Ling, Y.; Liu, Y.; Peng, J.; Zhong, S.; Huang, Y.; Liu, T.; Cheng, Y.; Zhang, Y.; Jiang, L.; Jiang, M. Analysis of the minimum ecological water level and water surplus and deficit in Bosten Lake. Environ. Eng. 2020, 38, 26–32+60. (In Chinese) [Google Scholar] [CrossRef]
- Wang, C.; Zhou, L.; Dai, B.; Gu, C.; Jiang, Z. The impacts of water level fluctuations between wet and dry seasons on taxonomic and functional diversity of fish communities in the ecotone floodplain of Lake Caizi. J. Lake Sci. 2019, 31, 1403–1414. [Google Scholar]
- Cao, Y. Study on Impact Factor and Technique of Vegetation Restoration for Flood Beaches Wetlands along Yangtze River. Ph.D. Thesis, Nanjing Normal University, Nanjing, China, 2007. [Google Scholar]
- Wang, H.-Z.; Wang, H.-J.; Liang, X.-M.; Ni, L.-Y.; Liu, X.-Q.; Cui, Y.-D. Empirical modelling of submersed macrophytes in Yangtze lakes. Ecol. Model. 2005, 188, 483–491. [Google Scholar] [CrossRef]
- Fang, H.; Zhu, Y.; Wang, C.; Xu, G.; Li, Y.; Wang, Z.; Aljawzi, A.A. Multiple-criteria determination and preventive measures of river ecological water level in the Northern Jiangsu plain. Watershed Ecol. Environ. 2023, 5, 64–72. [Google Scholar] [CrossRef]
- Hao, Z.; Xinguo, L.; Kai, Y.; Bin, L. Wetland Landscape Change in Small Lakes of Bosten Lake. Acta Bot. Boreal.-Occident. Sin. 2017, 36, 2533–2540. [Google Scholar]
- Zhang, X.; Wan, A.; Wang, H.; Zhu, L.; Yin, J.; Liu, Z.; Yu, D. The overgrowth of Zizania latifolia in a subtropical floodplain lake: Changes in its distribution and possible water level control measures. Ecol. Eng. 2016, 89, 114–120. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Liu, F.; Li, W. Morphological changes and resource allocation of Zizania latifolia (Griseb.) Stapf in response to different submergence depth and duration. Flora-Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 279–284. [Google Scholar] [CrossRef]
- Jia, Q.; Zhou, L.; Xie, Y.; Zhou, G. Study on biomass dynamics of Phragmites communis community in Panjin wetland. J. Meteorol. Environ. 2006, 22, 25–29. [Google Scholar]
- Qiao, B.; He, T.; Wu, C.; Su, Z. Study on Growth Characteristics of Reed and Its Habitat Soil Factors in Typical Lake Wetland of Yinchuan. Acta Bot. Boreal.-Occident. Sin. 2017, 37, 569–577. [Google Scholar]
- Yang, Y.; Du, C.; Zhang, J.; Yu, G.; Chen, H.; Qian, Z. Determination of lowest ecological water level in Dongting Lake area. Water Resour. Prot. 2019, 35, 89–94. [Google Scholar]
- Gao, X.; Hu, Z.; Li, X.; Zhou, X. Lowest Ecological Water Level of Yuecheng Reservoir. Haihe River Water Conserv. 2023, 18–21. (In Chinese) [Google Scholar]
- Liu, X.; Yang, Z.; Yuan, S.; Wang, H. A novel methodology for the assessment of water level requirements in shallow lakes. Ecol. Eng. 2017, 102, 31–38. [Google Scholar] [CrossRef]
- Zhang, X. Water Level Fluctuation Requirements of Plants in the Yangtze Floodplain Lakes. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2013. [Google Scholar]
- Wang, R.; He, L.; Zhang, M.; Cao, T.; Zhang, X.; Liu, Y.; Ni, L.; Ge, G. Factors on seed germination, tuber sprout and plant growth of Vallisnera species in China. J. Lake Sci. 2021, 33, 1315–1333. [Google Scholar]
- Schultz, R.E.; Pett, L. Plant community effects on CH4 fluxes, root surface area, and carbon storage in experimental wetlands. Ecol. Eng. 2018, 114, 96–103. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, Z.; Balke, T.; Zhang, L.; Bouma, T.J. Effects of sediment disturbance regimes on Spartina seedling establishment: Implications for salt marsh creation and restoration. Limnol. Oceanogr. 2018, 63, 647–659. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.; Verhoeven, J.T.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Change Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef]
- Hultine, K.R.; Froend, R.; Blasini, D.; Bush, S.E.; Karlinski, M.; Koepke, D.F. Hydraulic traits that buffer deep-rooted plants from changes in hydrology and climate. Hydrol. Process. 2020, 34, 209–222. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Xia, S.; Zhang, G.; Li, S.; Yu, D.; Yu, X. Hydrologic-induced concentrated soil nutrients and improved plant growth increased carbon storage in a floodplain wetland over wet-dry alternating zones. Sci. Total Environ. 2022, 822, 153512. [Google Scholar] [CrossRef] [PubMed]
- Kath, J.; Le Brocque, A.; Leyer, I.; Mosner, E. Hydrological and land use determinants of E ucalyptus camaldulensis occurrence in floodplain wetlands. Austral Ecol. 2014, 39, 643–655. [Google Scholar] [CrossRef]








| Month | Growth Stage | Water Level Requirement | Water Level Range |
|---|---|---|---|
| February–March | Sprouting Period | Minimum Ecological Water Level | 32.50 m |
| April–May | Seedling Growth Period | Gradual increase in water level, with an increase rate of less than 0.5 m per month | 32.50~33.00 m |
| June–July | Growth and Spread Period | It is recommended to keep a high water level, however, the rate of escalation should not surpass 1.00 m monthly | 33.50~34.00 m |
| August–September | Maturity Period | Ensure the water level remains consistently high, being mindful not to surpass the designated cautionary mark for water levels. | 33.50~34.00 m |
| October–November | Seed Propagation Period | Slow decrease in water level, with a decrease rate not exceeding 3 cm per day | 32.60~33.10 m |
| December–January | Dormant Period | Maintain low water level | 32.50 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, P.; Zhang, M.; Zhou, S. Research on the Reconstruction of Aquatic Vegetation Landscape in Coal Mining Subsidence Wetlands Based on Ecological Water Level. Water 2024, 16, 1339. https://doi.org/10.3390/w16101339
Luo P, Zhang M, Zhou S. Research on the Reconstruction of Aquatic Vegetation Landscape in Coal Mining Subsidence Wetlands Based on Ecological Water Level. Water. 2024; 16(10):1339. https://doi.org/10.3390/w16101339
Chicago/Turabian StyleLuo, Pingjia, Mengchu Zhang, and Shiyuan Zhou. 2024. "Research on the Reconstruction of Aquatic Vegetation Landscape in Coal Mining Subsidence Wetlands Based on Ecological Water Level" Water 16, no. 10: 1339. https://doi.org/10.3390/w16101339







