Liposomal Glutathione Augments Immune Defenses against Respiratory Syncytial Virus in Neonatal Mice Exposed in Utero to Ethanol
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- Fetal ethanol exposure promoted chronic oxidant stress in alveolar macrophages and systemically.
- Chronic oxidant stress resulted in immunosuppression of alveolar macrophages.
- In utero ethanol exposure impaired the capacity of pup alveolar macrophages to clear viruses.
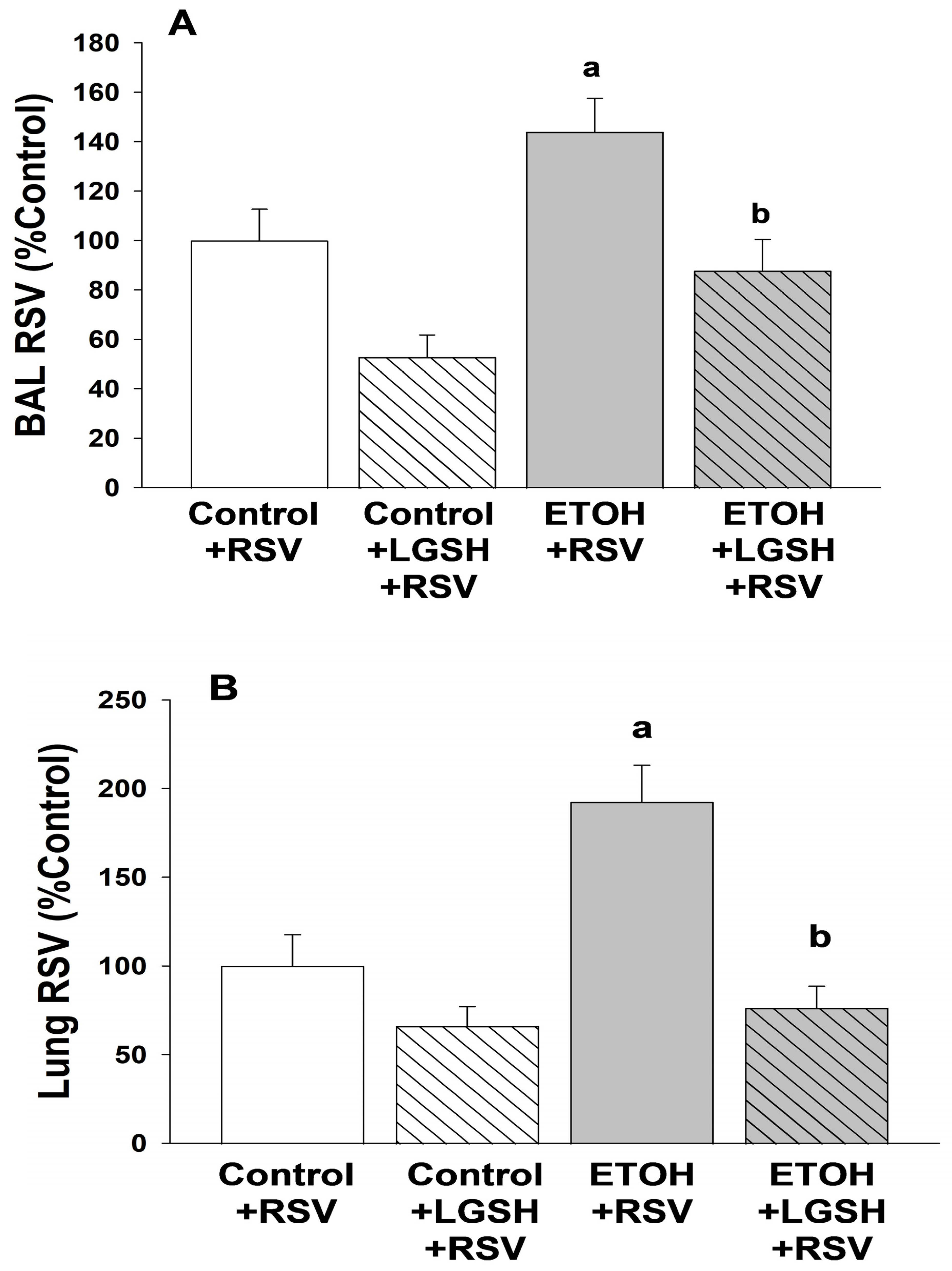
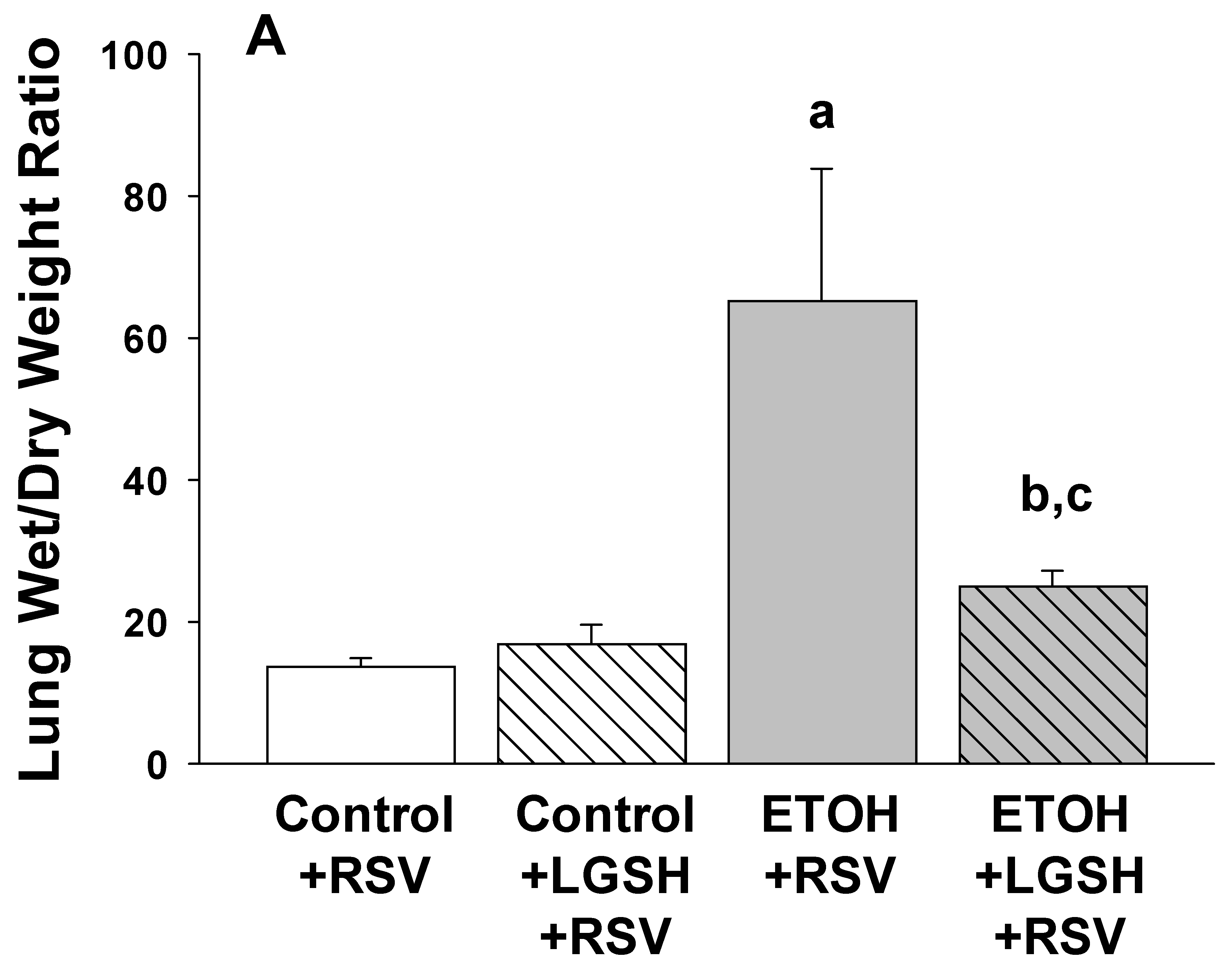
- Fetal ethanol exposure exacerbated lung respiratory syncytial virus infection and acute lung injury.
- Enteral treatments of the pup with liposomal glutathione normalized alveolar macrophage immune responses, lung viral infection, and acute lung injury.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | alveolar macrophage |
| AOC | antioxidant capacity |
| Arg-1 | arginase 1 |
| BAL | bronchoalveolar lavage |
| ETOH | ethanol |
| GSH | glutathione |
| 8-OHdG | 8-hydroxyguanosine |
| LGSH | liposomal glutathione |
| PFU | plaque forming units |
| PI | phagocytic index |
| PMNs | polymorphonuclear leukocytes |
| RFU | relative fluorescence unit |
| RSV | respiratory syncytial virus |
| TGFβ1 | transforming growth factor β1 |
References
- Gauthier, T.W. Prenatal Alcohol Exposure and the Developing Immune System. Alcohol Res. Curr. Rev. 2015, 37, 279–285. [Google Scholar]
- Gauthier, T.W.; Guidot, D.M.; Kelleman, M.S.; McCracken, C.E.; Brown, L.A. Maternal Alcohol Use During Pregnancy and Associated Morbidities in Very Low Birth Weight Newborns. Am. J. Med. Sci. 2016, 352, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.B.; Hjollund, N.H.; Jensen, T.K.; Bonde, J.P.; Andersson, A.M.; Kolstad, H.; Ernst, E.; Giwercman, A.; Skakkebaek, N.E.; Olsen, J. Alcohol consumption at the time of conception and spontaneous abortion. Am. J. Epidemiol. 2004, 160, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.; Wisborg, K.; Olsen, S.F.; Henriksen, T.B.; Secher, N.J. Moderate alcohol intake during pregnancy and the risk of stillbirth and death in the first year of life. Am. J. Epidemiol. 2002, 155, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.; Wisborg, K.; Olsen, S.F.; Henriksen, T.B.; Secher, N.J. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol Alcohol. 2002, 37, 87–92. [Google Scholar] [CrossRef]
- Albertsen, K.; Andersen, A.M.; Olsen, J.; Gronbaek, M. Alcohol consumption during pregnancy and the risk of preterm delivery. Am. J. Epidemiol. 2004, 159, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.; Olsen, S.F.; Secher, N.J. Does alcohol increase the risk of preterm delivery? Epidemiology 2000, 11, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.; Bakker, R.; Irving, H.; Jaddoe, V.W.; Malini, S.; Rehm, J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG 2011, 118, 1411–1421. [Google Scholar] [CrossRef]
- O’Callaghan, F.V.; O’Callaghan, M.; Najman, J.M.; Williams, G.M.; Bor, W. Maternal alcohol consumption during pregnancy and physical outcomes up to 5 years of age: A longitudinal study. Early Hum. Dev. 2003, 71, 137–148. [Google Scholar] [CrossRef]
- Liang, Y.; Harris, F.L.; Jones, D.P.; Brown, L.A. Alcohol induces mitochondrial redox imbalance in alveolar macrophages. Free Radic. Biol. Med. 2013, 65, 1427–1434. [Google Scholar] [CrossRef]
- Brown, S.D.; Brown, L.A. Ethanol (EtOH)-induced TGF-beta1 and reactive oxygen species production are necessary for EtOH-induced alveolar macrophage dysfunction and induction of alternative activation. Alcohol Clin. Exp. Res. 2012, 36, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Grunwell, J.R.; Yeligar, S.M.; Stephenson, S.; Ping, X.D.; Gauthier, T.W.; Fitzpatrick, A.M.; Brown, L.A.S. TGF-beta1 Suppresses the Type I IFN Response and Induces Mitochondrial Dysfunction in Alveolar Macrophages. J. Immunol. 2018, 200, 2115–2128. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.V.; McLaurin, K.; Ambrose, C.; Lee, H.C. Population-based trends and underlying risk factors for infant respiratory syncytial virus and bronchiolitis hospitalizations. PLoS ONE 2018, 13, e0205399. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Packnett, E.R.; Winer, I.H.; Larkin, H.; Oladapo, A.; Gonzales, T.; Wojdyla, M.; Goldstein, M.; Smith, V.C. RSV-related hospitalization and outpatient palivizumab use in very preterm (born at <29 wGA) infants: 2003-2020. Hum. Vaccin Immunother. 2022, 18, 2140533. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Balsells, E.; Wastnedge, E.; Singleton, R.; Rasmussen, Z.A.; Zar, H.J.; Rath, B.A.; Madhi, S.A.; Campbell, S.; Vaccari, L.C.; et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020416. [Google Scholar] [CrossRef] [PubMed]
- Odumade, O.A.; van Haren, S.D.; Angelidou, A. Implications of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Pandemic on the Epidemiology of Pediatric Respiratory Syncytial Virus Infection. Clin. Infect. Dis. 2022, 75 (Suppl. S1), S130–S135. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Kimura, H.; Kato, M.; Nako, Y.; Kozawa, K.; Morikawa, A. Respiratory syncytial virus enhances the expression of CD11b molecules and the generation of superoxide anion by human eosinophils primed with platelet-activating factor. Intervirology 2002, 45, 43–51. [Google Scholar] [CrossRef]
- Eddens, T.; Parks, O.B.; Williams, J.V. Neonatal Immune Responses to Respiratory Viruses. Front. Immunol. 2022, 13, 863149. [Google Scholar] [CrossRef]
- Fonseca, W.; Lukacs, N.W.; Ptaschinski, C. Factors Affecting the Immunity to Respiratory Syncytial Virus: From Epigenetics to Microbiome. Front. Immunol. 2018, 9, 226. [Google Scholar] [CrossRef]
- Paes, B. Respiratory Syncytial Virus in Otherwise Healthy Prematurely Born Infants: A Forgotten Majority. Am. J. Perinatol. 2018, 35, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A.; Anderson, E.J.; Wu, X.; Ambrose, C.S. Effects of Chronologic Age and Young Child Exposure on Respiratory Syncytial Virus Disease among US Preterm Infants Born at 32 to 35 Weeks Gestation. PLoS ONE 2016, 11, e0166226. [Google Scholar] [CrossRef]
- Bohmwald, K.; Espinoza, J.A.; Pulgar, R.A.; Jara, E.L.; Kalergis, A.M. Functional Impairment of Mononuclear Phagocyte System by the Human Respiratory Syncytial Virus. Front. Immunol. 2017, 8, 1643. [Google Scholar] [CrossRef] [PubMed]
- Kolli, D.; Gupta, M.R.; Sbrana, E.; Velayutham, T.S.; Chao, H.; Casola, A.; Garofalo, R.P. Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 502–515. [Google Scholar] [CrossRef]
- Castro, S.M.; Guerrero-Plata, A.; Suarez-Real, G.; Adegboyega, P.A.; Colasurdo, G.N.; Khan, A.M.; Garofalo, R.P.; Casola, A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am. J. Respir. Crit. Care Med. 2006, 174, 1361–1369. [Google Scholar] [CrossRef]
- Cho, H.Y.; Imani, F.; Miller-DeGraff, L.; Walters, D.; Melendi, G.A.; Yamamoto, M.; Polack, F.P.; Kleeberger, S.R. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 2009, 179, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.A.; Bailey, K.L.; Simet, S.M.; Warren, K.J.; Sweeter, J.M.; DeVasure, J.M.; Pavlik, J.A.; Sisson, J.H. Alcohol potentiates RSV-mediated injury to ciliated airway epithelium. Alcohol 2019, 80, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.J.; Poole, J.A.; Sweeter, J.M.; DeVasure, J.M.; Wyatt, T.A. An association between MMP-9 and impaired T cell migration in ethanol-fed BALB/c mice infected with respiratory syncytial virus-2A. Alcohol 2019, 80, 25–32. [Google Scholar] [CrossRef]
- Ackermann, M.R. Lamb model of respiratory syncytial virus-associated lung disease: Insights to pathogenesis and novel treatments. ILAR J. 2014, 55, 4–15. [Google Scholar] [CrossRef]
- Lazic, T.; Wyatt, T.A.; Matic, M.; Meyerholz, D.K.; Grubor, B.; Gallup, J.M.; Kersting, K.W.; Imerman, P.M.; Almeida-De-Macedo, M.; Ackermann, M.R. Maternal alcohol ingestion reduces surfactant protein A expression by preterm fetal lung epithelia. Alcohol 2007, 41, 347–355. [Google Scholar] [CrossRef]
- Johnson, J.K.; Harris, F.L.; Ping, X.D.; Gauthier, T.W.; Brown, L.A.S. Role of zinc insufficiency in fetal alveolar macrophage dysfunction and RSV exacerbation associated with fetal ethanol exposure. Alcohol 2019, 80, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.; Lagman, M.; Saing, T.; Singh, M.K.; Tudela, E.V.; Morris, D.; Anderson, J.; Daliva, J.; Ochoa, C.; Patel, N.; et al. Liposomal Glutathione Supplementation Restores TH1 Cytokine Response to Mycobacterium tuberculosis Infection in HIV-Infected Individuals. J. Interferon Cytokine Res. 2015, 35, 875–887. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Nguyen, T.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Medina, A.; et al. Effects of Oral Liposomal Glutathione in Altering the Immune Responses Against Mycobacterium tuberculosis and the Mycobacterium bovis BCG Strain in Individuals With Type 2 Diabetes. Front. Cell. Infect. Microbiol. 2021, 11, 657775. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Sathananthan, A.; Venketaraman, V. The Effects of Oral Liposomal Glutathione and In Vitro Everolimus in Altering the Immune Responses against Mycobacterium bovis BCG Strain in Individuals with Type 2 Diabetes. Biomol. Concepts 2021, 12, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.W.; Drury, J.A. Reduction of oxidative stress marker in lung fluid of preterm infants after administration of intra-tracheal liposomal glutathione. Biol. Neonate 2005, 87, 178–180. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Moore, M.L.; Rudd, B.D.; Berlin, A.A.; Collins, R.D.; Olson, S.J.; Ho, S.B.; Peebles, R.S. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J. Pathol. 2006, 169, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Ping, X.D.; Harris, F.L.; Gauthier, T.W. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L824–L832. [Google Scholar] [CrossRef]
- Ping, X.D.; Harris, F.L.; Brown, L.A.; Gauthier, T.W. In vivo dysfunction of the term alveolar macrophage after in utero ethanol exposure. Alcohol Clin. Exp. Res. 2007, 31, 308–316. [Google Scholar] [CrossRef]
- Akison, L.K.; Kuo, J.; Reid, N.; Boyd, R.N.; Moritz, K.M. Effect of Choline Supplementation on Neurological, Cognitive, and Behavioral Outcomes in Offspring Arising from Alcohol Exposure During Development: A Quantitative Systematic Review of Clinical and Preclinical Studies. Alcohol Clin. Exp. Res. 2018, 42, 1591–1611. [Google Scholar] [CrossRef]
- Gupta, K.K.; Gupta, V.K.; Shirasaka, T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol Clin. Exp. Res. 2016, 40, 1594–1602. [Google Scholar] [CrossRef]
- Caputo, C.; Wood, E.; Jabbour, L. Impact of fetal alcohol exposure on body systems: A systematic review. Birth Defects Res. C Embryo Today 2016, 108, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gomutputra, P.; Wolgemuth, D.J.; Baxi, L. Effects of acute alcohol intoxication in the second trimester of pregnancy on development of the murine fetal lung. Am. J. Obs. Gynecol. 2007, 197, 269.e1–269.e4. [Google Scholar] [CrossRef] [PubMed]
- Sozo, F.; O’Day, L.; Maritz, G.; Kenna, K.; Stacy, V.; Brew, N.; Walker, D.; Bocking, A.; Brien, J.; Harding, R. Repeated ethanol exposure during late gestation alters the maturation and innate immune status of the ovine fetal lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L510–L518. [Google Scholar] [CrossRef] [PubMed]
- Lazic, T.; Sow, F.B.; Van Geelen, A.; Meyerholz, D.K.; Gallup, J.M.; Ackermann, M.R. Exposure to ethanol during the last trimester of pregnancy alters the maturation and immunity of the fetal lung. Alcohol 2011, 45, 673–680. [Google Scholar] [CrossRef]
- Gauthier, T.W.; Manar, M.H.; Brown, L.A.S. Is maternal alcohol use a risk factor for early-onset sepsis in the premature newborn? Alcohol 2004, 33, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P., Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P., Jr.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Libster, R.; Ferolla, F.M.; Hijano, D.R.; Acosta, P.L.; Erviti, A.; Polack, F.P.; Network, I.R. Alcohol during pregnancy worsens acute respiratory infections in children. Acta Paediatr. 2015, 104, e494–e499. [Google Scholar] [CrossRef]
- Bailey, K.L.; Wyatt, T.A.; Katafiasz, D.M.; Taylor, K.W.; Heires, A.J.; Sisson, J.H.; Romberger, D.J.; Burnham, E.L. Alcohol and Cannabis Use Alter Pulmonary Innate Immunity. Alcohol 2018, 80, 131–138. [Google Scholar] [CrossRef]
- Price, M.E.; Gerald, C.L.; Pavlik, J.A.; Schlichte, S.L.; Zimmerman, M.C.; Devasure, J.M.; Wyatt, T.A.; Sisson, J.H. Loss of cAMP-dependent stimulation of isolated cilia motility by alcohol exposure is oxidant dependent. Alcohol 2018, 80, 91–98. [Google Scholar] [CrossRef]
- Ren, J.; Liu, G.; Go, J.; Kolli, D.; Zhang, G.; Bao, X. Human metapneumovirus M2-2 protein inhibits innate immune response in monocyte-derived dendritic cells. PLoS ONE 2014, 9, e91865. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Takeuchi, O.; Kato, H.; Kumar, H.; Matsui, K.; Morii, E.; Aozasa, K.; Kawai, T.; Akira, S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 2007, 27, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Brewah, Y.A.; Delaney, T.; Welliver, T.; Burwell, T.; Benjamin, E.; Kuta, E.; Kozhich, A.; McKinney, L.; Suzich, J.; et al. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2008, 198, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, K.M.; Egana, L.; Orend, J.G.; Resetar, E.; Anderson, K.B.; Patel, R.; Empey, K.M. Alveolar macrophages support interferon gamma-mediated viral clearance in RSV-infected neonatal mice. Respir. Res. 2015, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.P.; Kolli, D.; Casola, A. Respiratory syncytial virus infection: Mechanisms of redox control and novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 186–217. [Google Scholar] [CrossRef] [PubMed]
- El Saleeby, C.M.; Bush, A.J.; Harrison, L.M.; Aitken, J.A.; Devincenzo, J.P. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J. Infect. Dis. 2011, 204, 996–1002. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Liu, T.; Castro, S.M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009, 41, 348–357. [Google Scholar] [CrossRef]
- Kachour, N.; Beever, A.; Owens, J.; Cao, R.; Kolloli, A.; Kumar, R.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; et al. Liposomal Glutathione Helps to Mitigate Mycobacterium tuberculosis Infection in the Lungs. Antioxidants 2022, 11, 673. [Google Scholar] [CrossRef]
- Beever, A.; Kachour, N.; Owens, J.; Sasaninia, K.; Kolloli, A.; Kumar, R.; Ramasamy, S.; Sisliyan, C.; Khamas, W.; Subbian, S.; et al. L-GSH Supplementation in Conjunction With Rifampicin Augments the Treatment Response to Mycobacterium tuberculosis in a Diabetic Mouse Model. Front. Pharmacol. 2022, 13, 879729. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauthier, T.W.; Ping, X.-D.; Harris, F.L.; Brown, L.A.S. Liposomal Glutathione Augments Immune Defenses against Respiratory Syncytial Virus in Neonatal Mice Exposed in Utero to Ethanol. Antioxidants 2024, 13, 137. https://doi.org/10.3390/antiox13020137
Gauthier TW, Ping X-D, Harris FL, Brown LAS. Liposomal Glutathione Augments Immune Defenses against Respiratory Syncytial Virus in Neonatal Mice Exposed in Utero to Ethanol. Antioxidants. 2024; 13(2):137. https://doi.org/10.3390/antiox13020137
Chicago/Turabian StyleGauthier, Theresa W., Xiao-Du Ping, Frank L. Harris, and Lou Ann S. Brown. 2024. "Liposomal Glutathione Augments Immune Defenses against Respiratory Syncytial Virus in Neonatal Mice Exposed in Utero to Ethanol" Antioxidants 13, no. 2: 137. https://doi.org/10.3390/antiox13020137




