Effects of Dietary Callicarpa nudiflora Aqueous Extract Supplementation on Growth Performance, Growth Hormone, Antioxidant and Immune Function, and Intestinal Health of Broilers
Abstract
:1. Introduction
2. Materials and Methods
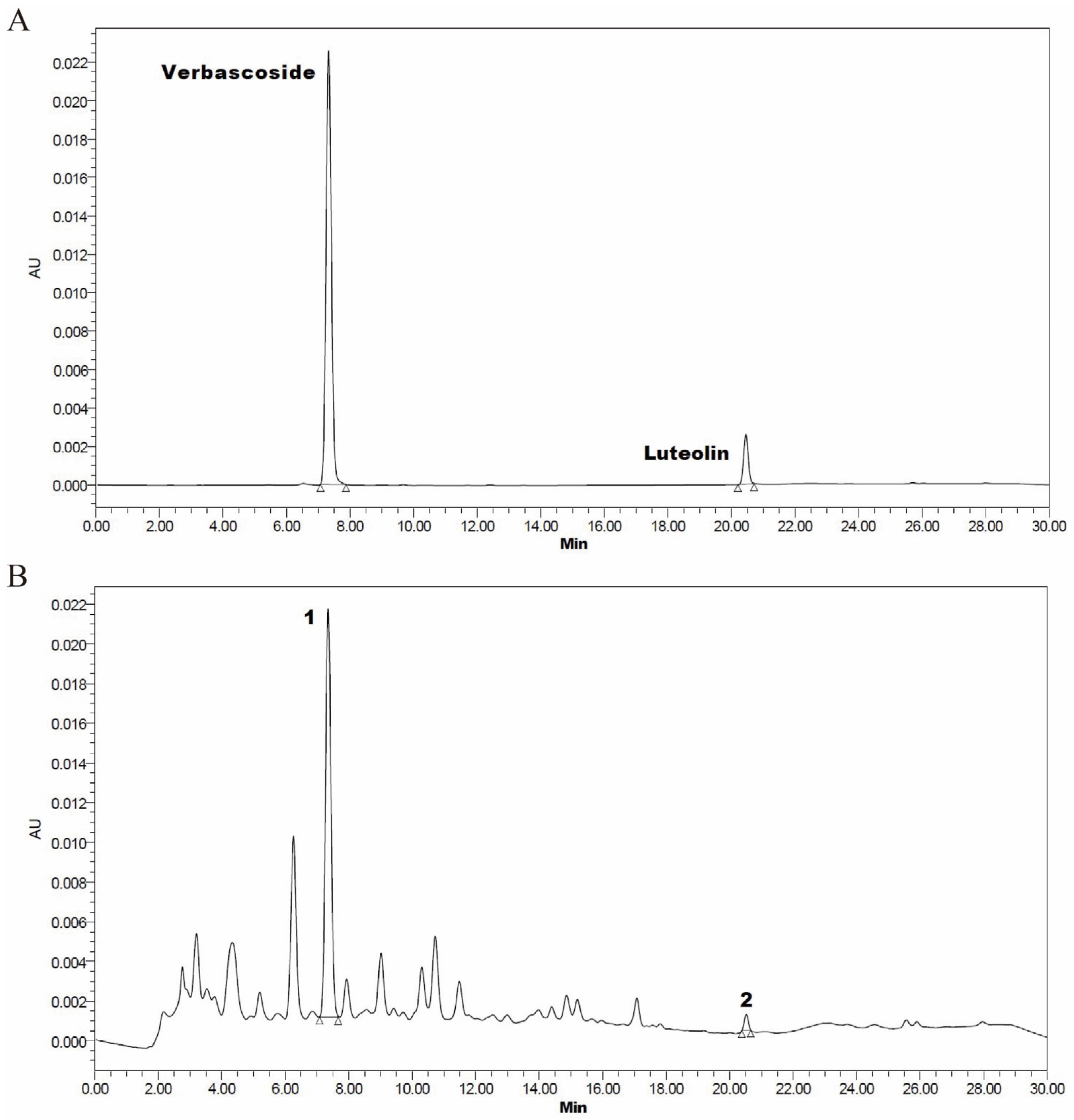
2.1. Callicarpa nudiflora Water Extraction and Chemical Components Determination
2.2. Birds, Experimental Design, and Diets
2.3. Growth Performance
2.4. Sample Collection
2.5. Determination of Serum Growth Hormone and Immune and Antioxidant Parameters
2.6. Measurements of Jejunal Morphology
2.7. Tissue RNA Extraction and qRT-PCR Analysis
2.8. 16S rRNA Sequencing and Gut Microbiota Analysis
2.9. Statistical Analysis
3. Results
3.1. Growth Performance of Broilers
3.2. Immune Organ Indices and Serum Immunoglobulin and Cytokines Level
3.3. Serum Antioxidant Parameters in Broilers
3.4. Serum Growth Hormone in Broilers
3.5. Immune-Related Gene Expression in Liver and Jejunum
3.6. Antioxidant-Related Gene Expression in Liver and Jejunum
3.7. Growth-Hormone-Related Factor Gene Expression in Liver and Jejunum
3.8. Intestinal Morphology and Genes mRNA Levels of Barrier Function and Nutrient Transporter
3.9. Bacterial Community Diversity and Composition of Cecal Microbiota
3.10. Functional Prediction of Cecal Microbes
3.11. Correlation Analysis between Cecal Microbes and Different Indicators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Yang, Z.; Zhao, C.; Tang, X.; Jiang, Q.; Yin, Y. A comprehensive review on natural phenolic compounds as alternatives to in-feed antibiotics. Sci. China Life Sci. 2023, 66, 1518–1534. [Google Scholar] [CrossRef] [PubMed]
- Jeni, R.E.; Dittoe, D.K.; Olson, E.G.; Lourenco, J.; Seidel, D.S.; Ricke, S.C.; Callaway, T.R. An overview of health challenges in alternative poultry production systems. Poult. Sci. 2021, 100, 101173. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.; Huque, K.S.; Kamaruddin, K.M.; Beg, M. Global restriction of using antibiotic growth promoters and alternative strategies in poultry production. Sci. Prog. 2018, 101, 52–75. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.I.; Oladokun, S.; Santin, E. Effect of organic acids-essential oils blend and oat fiber combination on broiler chicken growth performance, blood parameters, and intestinal health. Anim. Nutr. 2021, 7, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, G.; Wang, Q.; Tian, J.; Feng, X.; Zhang, Q.; Gong, L. Effects of dietary Astragalus membranaceus and Codonopsis pilosula extracts on growth performance, antioxidant capacity, immune status, and intestinal health in broilers. Front. Vet. Sci. 2023, 10, 1302801. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Shao, J.; Wang, G.; Wen, R.; Tian, J.; Hou, L. Callicarpa nudiflora Hook. & Arn.: A comprehensive review of its phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 264, 113123. [Google Scholar] [PubMed]
- Tu, Y.; Sun, L.; Guo, M.; Chen, W. The medicinal uses of Callicarpa L. in Traditional Chinese Medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2013, 146, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Kang, X.; Li, X.; Wu, Y.; Xiao, J.; Ye, Y.; Yang, J.; Yang, Y.; Liu, H. Study on The Anti-Inflammatory Effects of Callicarpa nudiflora Based on The Spectrum–Effect Relationship. Front. Pharmacol. 2022, 12, 806808. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Pan, J.; Ma, K. Verbascoside: A neuroprotective phenylethanoid glycosides with anti-depressive properties. Phytomedicine 2023, 120, 155027. [Google Scholar] [CrossRef]
- Rossi, R.; Mainardi, E.; Vizzarri, F.; Corino, C. Verbascoside-Rich Plant Extracts in Animal Nutrition. Antioxidants 2023, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, Q.; Wu, L. The pharmacokinetic property and pharmacological activity of acteoside: A review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Miceli, N.; D’Arrigo, M.; Marino, A.; Ekiert, H. Antioxidant Potential and Enhancement of Bioactive Metabolite Production in In Vitro Cultures of Scutellaria lateriflora L. by Biotechnological Methods. Molecules 2022, 27, 1140. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, Isoverbascoside, and Their Derivatives Recovered from Olive Mill Wastewater as Possible Food Antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shao, J.; Zhou, Q.; Chen, Y.; Tian, J.; Hou, L. Exploration of the mechanisms of Callicarpa nudiflora Hook. et Arn against influenza A virus (H1N1) infection. Phytomedicine 2024, 123, 155240. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ma, L.; Yi, B.; Zhang, M.; Feng, S.; Tian, L. Antidiabetic activity of Callicarpa nudiflora extract in type 2 diabetic rats via activation of the AMPK-ACC pathway. Asian Pac. J. Trop. Biomed. 2019, 9, 456. [Google Scholar]
- Shao, J.; Chen, W.K.; Ma, S.C.; Luo, Y.H. Stimultaneous determination of five flavonoids in Callicarpa nudiflora by UPLC. Chin. Tradit. Herb. Drugs 2014, 45, 1473–1476. [Google Scholar]
- Yao, C.; Dai, S.; Wang, C.; Fu, K.; Wu, R.; Zhao, X.; Yao, Y.; Li, Y. Luteolin as a potential hepatoprotective drug: Molecular mechanisms and treatment strategies. Biomed. Pharmacother. 2023, 167, 115464. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-kappaB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Dong, R.; Huang, K.; Wang, C.; Gu, J.; Luo, H.; Liu, K.; Wu, J.; Sun, H.; et al. Luteolin ameliorates LPS-induced acute liver injury by inhibiting TXNIP-NLRP3 inflammasome in mice. Phytomedicine 2021, 87, 153586. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Pillay, C.; Nyaga, M.M.; Sabiu, S. Poultry gut health—Microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. J. Anim. Sci. Biotechnol. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.; Ojeda, P.; Pierre, J.; Miyoshi, J.; Sontag, T.; Cham, C.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469.e5. [Google Scholar] [CrossRef]
- Ali, A. Flavonoids: Health Promoting Phytochemicals for Animal Production—A Review. J. Anim. Health Prod. 2015, 3, 6–13. [Google Scholar]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Li, X.M.; Zhou, X.X.; Wang, Y.; Lai, W.Y.; Liu, Y.; Luo, Y.C.; Zhang, J.Q. The Wound Healing Effect of Callicarpa nudiflora in Scalded Rats. Evid.-Based Complement. Altern. Med. 2019, 2019, 1860680. [Google Scholar]
- Shi, Y.; Wu, C.; Chen, Y.; Liu, W.; Feng, F.; Xie, N. Comparative analysis of three Callicarpa herbs using high performance liquid chromatography with diode array detector and electrospray ionization-trap mass spectrometry method. J. Pharm. Biomed. Anal. 2012, 75C, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Nong, K.; Qin, X.; Liu, Z.; Wang, Z.; Wu, Y.; Zhang, B.; Chen, W.; Fang, X.; Liu, Y.; Wang, X.; et al. Potential effects and mechanism of flavonoids extract of Callicarpa nudiflora Hook on DSS-induced colitis in mice. Phytomedicine 2024, 128, 155523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Zhang, Y.; Chen, W. Response of total phenols, flavonoids, minerals, and amino acids of four edible fern species to four shading treatments. PeerJ 2020, 8, e8354. [Google Scholar] [CrossRef]
- National, R.C. Nutrient Requirements of Poultry: Ninth Revised Edition; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Liu, M.; Chen, R.; Wang, T.; Ding, Y.; Zhang, Y.; Huang, G.; Huang, J.; Qu, Q.; Lv, W.; Guo, S. Dietary Chinese herbal mixture supplementation improves production performance by regulating reproductive hormones, antioxidant capacity, immunity, and intestinal health of broiler breeders. Poult. Sci. 2024, 103, 103201. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, J.; Li, Y.; Ding, Y.; Lian, J.; Dong, Q.; Qu, Q.; Lv, W.; Guo, S. Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers. Poult. Sci. 2023, 102, 102714. [Google Scholar] [CrossRef]
- Liu, M.; Huang, J.; Ma, M.; Huang, G.; Zhang, Y.; Ding, Y.; Qu, Q.; Lv, W.; Guo, S. Effects of dietary Chinese herbal mixtures on productive performance, egg quality, immune status, caecal and offspring meconial microbiota of Wenchang breeder hens. Front. Vet. Sci. 2023, 10, 1320469. [Google Scholar] [CrossRef]
- Steiner, T.; Shah, S.B.A. Phytogenic Feed Additives in Animal Nutrition. In Medicinal and Aromatic Plants of the World: Scientific, Production, Commercial and Utilization Aspects; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1, pp. 403–423. [Google Scholar]
- Shirani, V.; Jazi, V.; Toghyani, M.; Ashayerizadeh, A.; Sharifi, F.; Barekatain, R. Pulicaria gnaphalodes powder in broiler diets: Consequences for performance, gut health, antioxidant enzyme activity, and fatty acid profile. Poult. Sci. 2019, 98, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Dosu, G.; Obanla, T.O.; Zhang, S.; Sang, S.; Adetunji, A.O.; Fahrenholz, A.C.; Ferket, P.R.; Nagabhushanam, K.; Fasina, Y.O. Supplementation of ginger root extract into broiler chicken diet: Effects on growth performance and immunocompetence. Poult. Sci. 2023, 102, 102897. [Google Scholar] [CrossRef]
- Mehrparvar, M.; Mazhari, M.; Esmaeilipour, O.; Sami, M. Effect of Lipia citridora leaves powder on growth performance, carcass traits, blood metabolites and meat quality of broilers. Iran. J. Vet. Med. 2016, 10, 307–317. [Google Scholar]
- Hassan, M.; Wang, Y.; Rajput, S.A.; Shaukat, A.; Yang, P.; Farooq, M.Z.; Cheng, Q.; Ali, M.; Mi, X.; An, Y.; et al. Ameliorative Effects of Luteolin and Activated Charcoal on Growth Performance, Immunity Function, and Antioxidant Capacity in Broiler Chickens Exposed to Deoxynivalenol. Toxins 2023, 15, 478. [Google Scholar] [CrossRef]
- Kuhn, E.R.; Vleurick, L.; Edery, M.; Decuypere, E.; Darras, V.M. Internalization of the chicken growth hormone receptor complex and its effect on biological functions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.T.; Kunhareang, S.; Duangjinda, M. Association of Chicken Growth Hormones and Insulin-like Growth Factor Gene Polymorphisms with Growth Performance and Carcass Traits in Thai Broilers. Asian-Australas. J. Anim. Sci. 2015, 28, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Fan, H.; Zhang, B.; Xing, K.; Guo, Y. Dietary genistein supplementation for breeders and their offspring improves the growth performance and immune function of broilers. Sci. Rep. 2018, 8, 5161. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, B.; Liu, H.; Chen, S.; Zhang, J.; Wang, T.; Wang, C. Dietary rutin improves the antidiarrheal capacity of weaned piglets by improving intestinal barrier function, antioxidant capacity and cecal microbiota composition. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.; Uzunboy, S.; Capanoglu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2021, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ma, J.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, B. Artemisia annua L. aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers. Ital. J. Anim. Sci. 2020, 19, 399–409. [Google Scholar] [CrossRef]
- Chen, D.; Shen, F.; Liu, J.; Tang, H.; Teng, X.; Yang, F.; Liu, H. Luteolin enhanced antioxidant capability and induced pyroptosis through NF-κB/NLRP3/Caspase-1 in splenic lymphocytes exposure to ammonia. Sci. Total Environ. 2024, 919, 170699. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Fidanza, P.; Potapovich, A.I.; Kostyuk, V.A.; De Luca, C.; Mikhal’Chik, E.; Korkina, L.G. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxid. Redox Signal. 2012, 16, 314–328. [Google Scholar] [CrossRef]
- Quirantes-Pine, R.; Herranz-Lopez, M.; Funes, L.; Borras-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 2013, 20, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Lee, D.-B. Nelumbo nucifera leaves protects hydrogen peroxide-induced hepatic damage via antioxidant enzyme and HO-1/Nrf2 activation. Food Funct. 2015, 6, 1911–1918. [Google Scholar] [CrossRef]
- Schat, K.A. The Importance of the Bursa of Fabricius, B Cells and T Cells for the Pathogenesis of Marek’s Disease: A Review. Viruses 2022, 14, 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Zhang, J.L.; Gao, Y.H.; Wang, Q.H.; Li, S.; Wang, X.L.; Xu, S.W. Effect of oxygen free radicals and nitric oxide on apoptosis of immune organ induced by selenium deficiency in chickens. Biometals 2013, 26, 355–365. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, J.; Chang, Y.; Wang, S.; Shi, M.; Miao, Z. Effects of Chinese yam polysaccharides on the immune function and serum biochemical indexes of broilers. Front. Vet. Sci. 2022, 9, 1013888. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Q.; Qin, Y.; Si, W.; Zhang, H.; Zhang, J. The Effect of Epimedium Isopentenyl Flavonoids on the Broiler Gut Health Using Microbiomic and Metabolomic Analyses. Int. J. Mol. Sci. 2023, 24, 7646. [Google Scholar] [CrossRef]
- Rothstein, A.; Rifkin, I. Immunologically Active Autoantigens: The Role of Toll-like Receptors in the Development of Chronic Inflammatory Disease. Annu. Rev. Immunol. 2007, 25, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Rutz, S.; Ouyang, W. Regulation of Interleukin-10 Expression. In Regulation of Cytokine Gene Expression in Immunity and Diseases; Springer: Berlin/Heidelberg, Germany, 2016; Volume 941, pp. 89–116. [Google Scholar]
- Ma, H.; Qin, S.; Zhao, S. Osteoarthritis is Prevented in Rats by Verbascoside via Nuclear Factor kappa B (NF-kappaB) Pathway Downregulation. Med. Sci. Monit. 2020, 26, e921276. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Esposito, E.; Di Paola, R.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Effects of verbascoside biotechnologically produced by Syringa vulgaris plant cell cultures in a rodent model of colitis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 380, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, F.; Lei, X.; Yao, Y.; Liao, G.; Liu, Z.; Jiang, Z.; Zhou, H.; Wu, P. 3,4-seco-Labdane diterpenoids from the leaves of Callicarpa nudiflora with anti-inflammatory effects. Chin. J. Nat. Med. 2019, 17, 707–712. [Google Scholar] [PubMed]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Xie, Z.; Li, H.; Zhang, H.; Chen, F.; Xie, Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020, 99, 6606–6618. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, L.; Chen, W.; Xing, D.; Wu, X. Effects of Ilicis Chinensis folium extract supplementation on growth performance, serum parameters, intestinal morphology, and antioxidant capacity of broiler chickens. BMC Vet. Res. 2023, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, H.; Wen, X.; Ho, C.; Li, S. Citrus flavonoids and the intestinal barrier: Interactions and effects. Compr. Rev. Food Sci. Food Saf. 2020, 20, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Zhao, B.; Adams, S.; Yueli, F.; Rui, H.; Zhang, C.; Qin, G.; Hailong, J. Eleutheroside B increase tight junction proteins and anti-inflammatory cytokines expression in intestinal porcine jejunum epithelial cells (IPEC-J2). J. Anim. Physiol. Anim. Nutr. 2019, 103, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Romero Millan, L.; Persia, M. Effects of Protease, Phytase and a Bacillus sp. Direct-Fed Microbial on Nutrient and Energy Digestibility, Ileal Brush Border Digestive Enzyme Activity and Cecal Short-Chain Fatty Acid Concentration in Broiler Chickens. PLoS ONE 2014, 9, e101888. [Google Scholar] [CrossRef]
- Ye, J.; Gao, C.; Li, X.; Jin, C.; Wang, D.; Shu, G.; Wang, W.; Kong, X.; Yao, K.; Yan, H.; et al. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 2016, 7, 38681–38692. [Google Scholar] [CrossRef]
- Cushing, K.; Alvarado, D.M.; Ciorba, M.A. Butyrate and Mucosal Inflammation: New Scientific Evidence Supports Clinical Observation. Clin. Transl. Gastroenterol. 2015, 6, e108. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, F.; Yang, Y.; Lepp, D.; Yu, H.; Ruan, Z.; Yang, C.; Yin, Y.; Hou, Y.; Leeson, S.; et al. Dietary butyrate glycerides modulate intestinal microbiota composition and serum metabolites in broilers. Sci. Rep. 2018, 8, 4940. [Google Scholar] [CrossRef] [PubMed]
- Geirnaert, A.; Steyaert, A.; Eeckhaut, V.; Debruyne, B.; Arends, J.; Immerseel, F.; Boon, N.; Van de Wiele, T. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe 2014, 30, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Wang, J.; Yu, L.; Zhang, Q.; Chen, K.; Liu, B. Modulation of Growth Performance and Intestinal Microbiota in Chickens Fed Plant Extracts or Virginiamycin. Front. Microbiol. 2019, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.; Ursell, L.; Wegener Parfrey, L.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Esquivel Elizondo, S.; Ilhan, Z.; García-Peña, E.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef]
- Parada Venegas, D.; Fuente, M.; Landskron, G.; Gonzalez, M.; Quera, R.; Dijkstra, G.; Harmsen, H.; Faber, K.; Hermoso, M. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]






| Items | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | CNEL | CNEM | CNEH | T | L | Q | ||
| BW | ||||||||
| 21 day (g) | 796.9 b | 820.9 a | 816.1 a | 806.8 ab | 2.99 | 0.013 | 0.272 | 0.003 |
| 42 day (g) | 2293.3 b | 2360.4 a | 2345.4 a | 2316.7 ab | 8.85 | 0.026 | 0.423 | 0.005 |
| 1 to 21 day | ||||||||
| ADG (g) | 36.28 b | 37.45 a | 37.23 a | 36.77 ab | 0.14 | 0.009 | 0.247 | 0.002 |
| ADFI (g) | 54.05 | 53.35 | 52.11 | 52.99 | 0.28 | 0.098 | 0.073 | 0.145 |
| F/G | 1.49 a | 1.42 b | 1.40 b | 1.45 b | 0.01 | 0.002 | 0.015 | 0.001 |
| 22 to 42 day | ||||||||
| ADG (g) | 71.26 | 73.31 | 72.78 | 71.90 | 0.39 | 0.260 | 0.688 | 0.068 |
| ADFI (g) | 127.81 | 129.71 | 127.96 | 127.88 | 0.49 | 0.492 | 0.733 | 0.334 |
| F/G | 1.79 | 1.77 | 1.76 | 1.77 | 0.09 | 0.431 | 0.391 | 0.168 |
| 1 to 42 day | ||||||||
| ADG (g) | 53.77 b | 55.38 a | 55.01 a | 54.33 ab | 0.21 | 0.024 | 0.425 | 0.005 |
| ADFI (g) | 90.93 | 91.53 | 90.04 | 90.44 | 0.28 | 0.263 | 0.231 | 0.852 |
| F/G | 1.69 a | 1.65 b | 1.64 b | 1.66 ab | 0.01 | 0.019 | 0.060 | 0.008 |
| Items | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | CNEL | CNEM | CNEH | T | L | Q | ||
| 21 day | ||||||||
| Spleen index (%) | 0.075 b | 0.088 ab | 0.091 a | 0.093 a | 0.003 | 0.043 | 0.010 | 0.269 |
| Thymus index (%) | 0.227 b | 0.256 ab | 0.247 a | 0.296 a | 0.009 | 0.036 | 0.010 | 0.523 |
| Bursa index (%) | 0.261 | 0.291 | 0.267 | 0.282 | 0.011 | 0.774 | 0.714 | 0.749 |
| IgA (μg/mL) | 321.3 c | 380.7 a | 359.5 ab | 328.7 bc | 7.36 | 0.006 | 0.988 | 0.001 |
| IgM (μg/mL) | 843.0 b | 930.7 a | 869.0 ab | 897.2 ab | 12.66 | 0.047 | 0.259 | 0.163 |
| IL-6 (pg/mL) | 35.8 a | 33.3 ab | 32.3 b | 32.0 b | 0.51 | 0.020 | 0.004 | 0.218 |
| IL-10 (pg/mL) | 103.8 | 99.1 | 101.6 | 101.3 | 0.94 | 0.385 | 0.550 | 0.257 |
| TNF-α (pg/mL) | 108.0 a | 105.8 a | 106.2 a | 97.1 b | 1.12 | <0.001 | <0.001 | 0.033 |
| 42 day | ||||||||
| Spleen index (%) | 0.085 b | 0.120 a | 0.099 ab | 0.116 a | 0.005 | 0.033 | 0.067 | 0.306 |
| Thymus index (%) | 0.096 | 0.100 | 0.108 | 0.090 | 0.004 | 0.576 | 0.578 | 0.507 |
| Bursa index (%) | 0.146 | 0.178 | 0.157 | 0.167 | 0.008 | 0.560 | 0.834 | 0.238 |
| IgA (μg/mL) | 343.9 | 359.6 | 366.7 | 344.7 | 4.4 | 0.189 | 0.807 | 0.039 |
| IgM (μg/mL) | 829.8 | 860.8 | 836.7 | 848.5 | 8.6 | 0.369 | 0.150 | 0.400 |
| IL-6 (pg/mL) | 38.7 a | 32.9 b | 34.1 b | 30.7 b | 0.83 | 0.001 | <0.001 | 0.325 |
| IL-10 (pg/mL) | 87.0 b | 90.8 ab | 92.4 a | 90.6 ab | 0.72 | 0.036 | 0.035 | 0.035 |
| TNF-α (pg/mL) | 100.5 a | 94.8 b | 94.2 b | 86.8 c | 1.17 | <0.001 | <0.001 | 0.482 |
| Items | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | CNEL | CNEM | CNEH | T | L | Q | ||
| 21 day | ||||||||
| SOD (U/mL) | 186.6 b | 195.8 b | 258.3 a | 241.7 a | 7.92 | <0.001 | <0.001 | 0.226 |
| GSH-Px (μmol/L) | 43.4 | 47.1 | 43.8 | 45.5 | 1.26 | 0.733 | 0.792 | 0.693 |
| T-AOC (mmol/L) | 0.51 b | 0.55 b | 0.57 ab | 0.64 a | 0.02 | 0.012 | 0.001 | 0.651 |
| MDA (nmol/mL) | 9.8 a | 5.9 b | 6.9 b | 6.6 b | 0.50 | 0.016 | 0.029 | 0.042 |
| 42 day | ||||||||
| SOD (U/mL) | 202.5 c | 213.7 bc | 239.5 ab | 248.3 a | 5.94 | 0.010 | 0.001 | 0.907 |
| GSH-Px (μmol/L) | 31.6 b | 32.8 b | 41.5 a | 42.6 a | 1.52 | 0.005 | 0.001 | 0.973 |
| T-AOC (mmol/L) | 0.51 b | 0.59 a | 0.61 a | 0.62 a | 0.01 | 0.002 | 0.001 | 0.059 |
| MDA (nmol/mL) | 11.2 a | 6.2 b | 8.0 b | 6.8 b | 0.53 | 0.001 | 0.003 | 0.022 |
| Items | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | CNEL | CNEM | CNEH | T | L | Q | ||
| 21 day | ||||||||
| GH (ng/mL) | 18.7 | 18.3 | 19.4 | 18.9 | 0.21 | 0.267 | 0.194 | 0.831 |
| IGF-1 (ng/mL) | 154.2 b | 158.0 b | 175.9 a | 161.2 b | 2.43 | 0.003 | 0.029 | 0.021 |
| 42 day | ||||||||
| GH (ng/mL) | 15.4 c | 16.5 bc | 17.5 ab | 18.4 a | 0.30 | <0.001 | <0.001 | 0.905 |
| IGF-1 (ng/mL) | 165.6 | 159.8 | 170.3 | 169.1 | 3.24 | 0.691 | 0.487 | 0.733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Huang, G.; Lin, Y.; Huang, Y.; Xuan, Z.; Lun, J.; He, S.; Zhou, J.; Chen, X.; Qu, Q.; et al. Effects of Dietary Callicarpa nudiflora Aqueous Extract Supplementation on Growth Performance, Growth Hormone, Antioxidant and Immune Function, and Intestinal Health of Broilers. Antioxidants 2024, 13, 572. https://doi.org/10.3390/antiox13050572
Liu M, Huang G, Lin Y, Huang Y, Xuan Z, Lun J, He S, Zhou J, Chen X, Qu Q, et al. Effects of Dietary Callicarpa nudiflora Aqueous Extract Supplementation on Growth Performance, Growth Hormone, Antioxidant and Immune Function, and Intestinal Health of Broilers. Antioxidants. 2024; 13(5):572. https://doi.org/10.3390/antiox13050572
Chicago/Turabian StyleLiu, Mengjie, Gengxiong Huang, Yulin Lin, Yiwen Huang, Zhaoying Xuan, Jianchi Lun, Shiqi He, Jing Zhou, Xiaoli Chen, Qian Qu, and et al. 2024. "Effects of Dietary Callicarpa nudiflora Aqueous Extract Supplementation on Growth Performance, Growth Hormone, Antioxidant and Immune Function, and Intestinal Health of Broilers" Antioxidants 13, no. 5: 572. https://doi.org/10.3390/antiox13050572





