Periapical Lesions in Panoramic Radiography and CBCT Imaging—Assessment of AI’s Diagnostic Accuracy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition and Post-Processing
2.3. Multireader Evaluation
2.4. AI Evaluation
2.5. Statistical Evaluation
3. Results
3.1. Population
3.2. Diagnostic Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The Global Prevalence of Apical Periodontitis: A Systematic Review and Meta-Analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G. Bacteriological Studies of Necrotic Dental Pulps. Ph.D. Thesis, Umeå University, Umeå, Sweden, 1976. [Google Scholar]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Abbott, P.V. Classification, Diagnosis and Clinical Manifestations of Apical Periodontitis. Endod. Topics 2004, 8, 36–54. [Google Scholar] [CrossRef]
- Kirkevang, L.L.; Ørstavik, D.; Bahrami, G.; Wenzel, A.; Væth, M. Prediction of Periapical Status and Tooth Extraction. Int. Endod. J. 2017, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Martín-González, J.; Castellanos-Cosano, L. Endodontic Medicine: Connections between Apical Periodontitis and Systemic Diseases. Int. Endod. J. 2015, 48, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.F.; Rocha, A.L.; Castro, W.H.; Gelape, C.L.; Nunes, M.C.P.; Oliveira, S.R.; Travassos, D.V.; Silva, T.A. Dental Management for Patients Undergoing Heart Valve Surgery. J. Card. Surg. 2017, 32, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.N.; Nadgir, R.N.; Akman, A.S.; Saito, N.; Sekiya, K.; Kaneda, T.; Sakai, O. Periapical Lucency around the Tooth: Radiologic Evaluation and Differential Diagnosis. Radiographics 2013, 33, E15–E32. [Google Scholar] [CrossRef]
- Pope, O.; Sathorn, C.; Parashos, P. A Comparative Investigation of Cone-Beam Computed Tomography and Periapical Radiography in the Diagnosis of a Healthy Periapex. J. Endod. 2014, 40, 360–365. [Google Scholar] [CrossRef]
- Patel, S.; Dawood, A.; Mannocci, F.; Wilson, R.; Pitt Ford, T. Detection of Periapical Bone Defects in Human Jaws Using Cone Beam Computed Tomography and Intraoral Radiography. Int. Endod. J. 2009, 42, 507–515. [Google Scholar] [CrossRef]
- Chang, L.; Umorin, M.; Augsburger, R.A.; Glickman, G.N.; Jalali, P. Periradicular Lesions in Cancellous Bone Can Be Detected Radiographically. J. Endod. 2020, 46, 496–501. [Google Scholar] [CrossRef]
- Nardi, C.; Calistri, L.; Pradella, S.; Desideri, I.; Lorini, C.; Colagrande, S. Accuracy of Orthopantomography for Apical Periodontitis without Endodontic Treatment. J. Endod. 2017, 43, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Bueno, M.R.; Leles, C.R.; Azevedo, B.; Azevedo, J.R. Accuracy of Cone Beam Computed Tomography and Panoramic and Periapical Radiography for Detection of Apical Periodontitis. J. Endod. 2008, 34, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Calistri, L.; Grazzini, G.; Desideri, I.; Lorini, C.; Occhipinti, M.; Mungai, F.; Colagrande, S. Is Panoramic Radiography an Accurate Imaging Technique for the Detection of Endodontically Treated Asymptomatic Apical Periodontitis? J. Endod. 2018, 44, 1500–1508. [Google Scholar] [CrossRef]
- Nardi, C.; Calistri, L.; Pietragalla, M.; Vignoli, C.; Lorini, C.; Berti, V.; Mungai, F.; Colagrande, S. Electronic Processing of Digital Panoramic Radiography for the Detection of Apical Periodontitis. Radiologia Medica 2020, 125, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bender, I.B.; Seltzer, S. Roentgenographic and Direct Observation of Experimental Lesions in Bone: I. J. Endod. 2003, 29, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Arias, E.; Huang, Y.H.; Zhao, L.; Seelaus, R.; Patel, P.; Cohen, M. Virtual Surgical Planning and Three-Dimensional Printed Guide for Soft Tissue Correction in Facial Asymmetry. J. Craniofacial Surg. 2019, 30, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.C.; Buck, D.B.; Herrmann, J.; Hamdan, A.D.; Wyers, M.; Patel, V.I.; Fillinger, M.; Schermerhorn, M.L. Risk factors and consequences of persistent type II endoleaks. J. Vasc. Surg. 2016, 63, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, K.; Gandhi Babu, D.B.; Chaitanya, N.C.S.K.; Guduru, H.; Rembers, A.; Soni, P. Evaluation of Preference and Purpose of Utilisation of Cone Beam Computed Tomography (CBCT) Compared to Orthopantomogram (OPG) by Dental Practitioners—A Cross-Sectional Study. Pol. J. Radiol. 2017, 82, 248. [Google Scholar] [CrossRef] [PubMed]
- Kaasalainen, T.; Ekholm, M.; Siiskonen, T.; Kortesniemi, M. Dental Cone Beam CT: An Updated Review. Phys. Medica 2021, 88, 193–217. [Google Scholar] [CrossRef]
- Barnett, C.W.; Glickman, G.N.; Umorin, M.; Jalali, P. Interobserver and Intraobserver Reliability of Cone-Beam Computed Tomography in Identification of Apical Periodontitis. J. Endod. 2018, 44, 938–940. [Google Scholar] [CrossRef]
- Davies, A.; Mannocci, F.; Mitchell, P.; Andiappan, M.; Patel, S. The Detection of Periapical Pathoses in Root Filled Teeth Using Single and Parallax Periapical Radiographs versus Cone Beam Computed Tomography—A Clinical Study. Int. Endod. J. 2015, 48, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Lofthag-Hansen, S.; Huumonen, S.; Gröndahl, K.; Gröndahl, H.G. Limited Cone-Beam CT and Intraoral Radiography for the Diagnosis of Periapical Pathology. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 103, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Abella, F.; Patel, S.; Duran-Sindreu, F.; Mercadé, M.; Bueno, R.; Roig, M. Evaluating the Periapical Status of Teeth with Irreversible Pulpitis by Using Cone-Beam Computed Tomography Scanning and Periapical Radiographs. J. Endod. 2012, 38, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Mostafapoor, M.; Hemmatian, S. Evaluation of the Accuracy Values of Cone-Beam CT Regarding Apical Periodontitis: A Systematic Review and Meta-Analysis. Oral. Radiol. 2022, 38, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.; Spin-Neto, R.; Reibel, J.; Wenzel, A.; Kirkevang, L.L. Diagnostic Validity of Periapical Radiography and CBct for Assessing Periapical Lesions That Persist after Endodontic Surgery. Dentomaxillofacial Radiol. 2017, 46, 20170210. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.S.; Kim, J.E.; Hwang, J.J.; Han, S.S.; Kim, J.S.; Yi, W.J.; Park, I.W. Dmfr 50th Anniversary: Review Article Artificial Intelligence in Oral and Maxillofacial Radiology: What Is Currently Possible? Dentomaxillofacial Radiol. 2020, 50, 20200375. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, N.; Kazimierczak, W.; Serafin, Z.; Nowicki, P.; Nożewski, J.; Janiszewska-Olszowska, J. AI in Orthodontics: Revolutionizing Diagnostics and Treatment Planning—A Comprehensive Review. J. Clin. Med. 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Abesi, F.; Jamali, A.S.; Zamani, M. Accuracy of Artificial Intelligence in the Detection and Segmentation of Oral and Maxillofacial Structures Using Cone-Beam Computed Tomography Images: A Systematic Review and Meta-Analysis. Pol. J. Radiol. 2023, 88, e256. [Google Scholar] [CrossRef]
- Hajem, S.; Brogårdh-Roth, S.; Nilsson, M.; Hellén-Halme, K. CBCT of Swedish Children and Adolescents at an Oral and Maxillofacial Radiology Department. A Survey of Requests and Indications. Acta Odontol. Scand. 2020, 78, 38–44. [Google Scholar] [CrossRef]
- Orhan, K.; Bayrakdar, I.S.; Ezhov, M.; Kravtsov, A.; Özyürek, T. Evaluation of Artificial Intelligence for Detecting Periapical Pathosis on Cone-Beam Computed Tomography Scans. Int. Endod. J. 2020, 53, 680–689. [Google Scholar] [CrossRef]
- Issa, J.; Jaber, M.; Rifai, I.; Mozdziak, P.; Kempisty, B.; Dyszkiewicz-Konwińska, M. Diagnostic Test Accuracy of Artificial Intelligence in Detecting Periapical Periodontitis on Two-Dimensional Radiographs: A Retrospective Study and Literature Review. Medicina 2023, 59, 768. [Google Scholar] [CrossRef]
- Vujanovic, T.; Jagtap, R. Evaluation of Artificial Intelligence for Automatic Tooth and Periapical Pathosis Detection on Panoramic Radiography. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2023, 135, e51. [Google Scholar] [CrossRef]
- Zadrożny, Ł.; Regulski, P.; Brus-Sawczuk, K.; Czajkowska, M.; Parkanyi, L.; Ganz, S.; Mijiritsky, E. Artificial Intelligence Application in Assessment of Panoramic Radiographs. Diagnostics 2022, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Fenn Buderer, N.M. Statistical Methodology: I. Incorporating the Prevalence of Disease into the Sample Size Calculation for Sensitivity and Specificity. Acad. Emerg. Med. 1996, 3, 895–900. [Google Scholar] [CrossRef]
- Hicks, S.A.; Strümke, I.; Thambawita, V.; Hammou, M.; Riegler, M.A.; Halvorsen, P.; Parasa, S. On Evaluation Metrics for Medical Applications of Artificial Intelligence. Sci. Rep. 2022, 12, 5979. [Google Scholar] [CrossRef]
- Fayad, M.I.; Nair, M.; Levin, M.D.; Benavides, E.; Rubinstein, R.A.; Barghan, S.; Hirschberg, C.S.; Ruprecht, A. AAE and AAOMR Joint Position Statement Use of Cone Beam Computed Tomography in Endodontics 2015 Update. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.H.; Doi, C.; Yoda, N.; Astuti, E.R.; Sasaki, K. Current Applications and Development of Artificial Intelligence for Digital Dental Radiography. Dentomaxillofacial Radiol. 2022, 51, 20210197. [Google Scholar] [CrossRef]
- Musri, N.; Christie, B.; Ichwan, S.J.A.; Cahyanto, A. Deep Learning Convolutional Neural Network Algorithms for the Early Detection and Diagnosis of Dental Caries on Periapical Radiographs: A Systematic Review. Imaging Sci. Dent. 2021, 51, 237. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, H.; Zhao, Y.; Zhao, J.; Wang, Y. Dental Disease Detection on Periapical Radiographs Based on Deep Convolutional Neural Networks. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 649–661. [Google Scholar] [CrossRef]
- Pauwels, R.; Brasil, D.M.; Yamasaki, M.C.; Jacobs, R.; Bosmans, H.; Freitas, D.Q.; Haiter-Neto, F. Artificial Intelligence for Detection of Periapical Lesions on Intraoral Radiographs: Comparison between Convolutional Neural Networks and Human Observers. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 131, 610–616. [Google Scholar] [CrossRef]
- Hamdan, M.H.; Tuzova, L.; Mol, A.; Tawil, P.Z.; Tuzoff, D.; Tyndall, D.A. The Effect of a Deep-Learning Tool on Dentists’ Performances in Detecting Apical Radiolucencies on Periapical Radiographs. Dentomaxillofacial Radiol. 2022, 51, 20220122. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, S.S.; Kim, Y.H.; Lee, C.; Kim, I. Application of a Fully Deep Convolutional Neural Network to the Automation of Tooth Segmentation on Panoramic Radiographs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 635–642. [Google Scholar] [CrossRef] [PubMed]
- İçöz, D.; Terzioǧlu, H.; Özel, M.A.; Karakurt, R. Evaluation of an Artificial Intelligence System for the Diagnosis of Apical Periodontitis on Digital Panoramic Images. Niger. J. Clin. Pract. 2023, 26, 1085–1090. [Google Scholar] [CrossRef]
- Song, I.S.; Shin, H.K.; Kang, J.H.; Kim, J.E.; Huh, K.H.; Yi, W.J.; Lee, S.S.; Heo, M.S. Deep Learning-Based Apical Lesion Segmentation from Panoramic Radiographs. Imaging Sci. Dent. 2022, 52, 351. [Google Scholar] [CrossRef]
- Bayrakdar, I.S.; Orhan, K.; Çelik, Ö.; Bilgir, E.; Saǧlam, H.; Kaplan, F.A.; Görür, S.A.; Odabaş, A.; Aslan, A.F.; Różyło-Kalinowska, I. A U-Net Approach to Apical Lesion Segmentation on Panoramic Radiographs. Biomed. Res. Int. 2022, 2022, 7035367. [Google Scholar] [CrossRef]
- Çelik, B.; Savaştaer, E.F.; Kaya, H.I.; Çelik, M.E. The Role of Deep Learning for Periapical Lesion Detection on Panoramic Radiographs. Dentomaxillofacial Radiol. 2023, 52, 20230118. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.G.; Hillen, F.; Salloumis, M.; Sedaghat, A.R.; Niehues, S.M.; Quatela, O.; Hanken, H.; Smeets, R.; Beck-Broichsitter, B.; Rendenbach, C.; et al. Development of a Deep Learning Algorithm for Periapical Disease Detection in Dental Radiographs. Diagnostics 2020, 10, 430. [Google Scholar] [CrossRef]
- Waller, J.; O’connor, A.; Rafaat, E.; Amireh, A.; Dempsey, J.; Martin, C.; Umair, M. Applications and Challenges of Artificial Intelligence in Diagnostic and Interventional Radiology. Pol. J. Radiol. 2022, 87, e113. [Google Scholar] [CrossRef] [PubMed]
- Vinayahalingam, S.; Goey, R.S.; Kempers, S.; Schoep, J.; Cherici, T.; Moin, D.A.; Hanisch, M. Automated Chart Filing on Panoramic Radiographs Using Deep Learning. J. Dent. 2021, 115, 103864. [Google Scholar] [CrossRef]
- Başaran, M.; Çelik, Ö.; Bayrakdar, I.S.; Bilgir, E.; Orhan, K.; Odabaş, A.; Aslan, A.F.; Jagtap, R. Diagnostic Charting of Panoramic Radiography Using Deep-Learning Artificial Intelligence System. Oral. Radiol. 2022, 38, 1–7. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Zhao, J.; Yu, Y.; Li, X.; Shi, K.; Zhang, F.; Yu, F.; Shi, K.; Sun, Z.; et al. Artificial Intelligence in the Diagnosis of Dental Diseases on Panoramic Radiographs: A Preliminary Study. BMC Oral Health 2023, 23, 358. [Google Scholar] [CrossRef] [PubMed]
- Brignardello-Petersen, R. Artificial Intelligence System Seems to Be Able to Detect a High Proportion of Periapical Lesions in Cone-Beam Computed Tomographic Images. J. Am. Dent. Assoc. 2020, 151, e83. [Google Scholar] [CrossRef] [PubMed]
- Setzer, F.C.; Shi, K.J.; Zhang, Z.; Yan, H.; Yoon, H.; Mupparapu, M.; Li, J. Artificial Intelligence for the Computer-Aided Detection of Periapical Lesions in Cone-Beam Computed Tomographic Images. J. Endod. 2020, 46, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.T.; Zhu, Q.K.; Li, N.; Wang, Y.Q.; Deng, S.L.; Chen, H.P.; Shen, J.; Meng, L.Y.; Bian, Z. Clinically Oriented CBCT Periapical Lesion Evaluation via 3D CNN Algorithm. J. Dent. Res. 2024, 103, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, B.; Hadzic, A.; Jakse, N.; Bischof, H.; Stern, D. Automatic Detection of Periapical Osteolytic Lesions on Cone-Beam Computed Tomography Using Deep Convolutional Neuronal Networks. J. Endod. 2022, 48, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yan, H.; Setzer, F.C.; Shi, K.J.; Mupparapu, M.; Li, J. Anatomically Constrained Deep Learning for Automating Dental CBCT Segmentation and Lesion Detection. IEEE Trans. Autom. Sci. Eng. 2021, 18, 603–614. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhou, Z.; Zhou, Z.; Wu, X.; Li, Y.; Wang, S.; Liao, W.; Ying, S.; Zhao, Z. Artificial Intelligence for Caries and Periapical Periodontitis Detection. J. Dent. 2022, 122, 104107. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzade, S.; Laurentiu, T.; Bakhshandah, A.; Ibragimov, B.; Kvist, T.; Bjørndal, L.; Bjørndal, L.; Dawson, V.S.; Fransson, H.; Frisk, F.; et al. The Efficiency of Artificial Intelligence Methods for Finding Radiographic Features in Different Endodontic Treatments—A Systematic Review. Acta Odontol. Scand. 2023, 81, 422–435. [Google Scholar] [PubMed]
- Silva, V.K.S.; Vieira, W.A.; Bernardino, Í.M.; Travençolo, B.A.N.; Bittencourt, M.A.V.; Blumenberg, C.; Paranhos, L.R.; Galvão, H.C. Accuracy of Computer-Assisted Image Analysis in the Diagnosis of Maxillofacial Radiolucent Lesions: A Systematic Review and Meta-Analysis. Dentomaxillofacial Radiol. 2020, 49, 20190204. [Google Scholar] [CrossRef]
- Badr, F.F.; Jadu, F.M. Performance of Artificial Intelligence Using Oral and Maxillofacial CBCT Images: A Systematic Review and Meta-Analysis. Niger. J. Clin. Pract. 2022, 25, 1918–1927. [Google Scholar] [CrossRef]
- Sadr, S.; Mohammad-Rahimi, H.; Motamedian, S.R.; Zahedrozegar, S.; Motie, P.; Vinayahalingam, S.; Dianat, O.; Nosrat, A. Deep Learning for Detection of Periapical Radiolucent Lesions: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy. J. Endod. 2023, 49, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Abesi, F.; Maleki, M.; Zamani, M. Diagnostic Performance of Artificial Intelligence Using Cone-Beam Computed Tomography Imaging of the Oral and Maxillofacial Region: A Scoping Review and Meta-Analysis. Imaging Sci. Dent. 2023, 53, 101. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Rohban, M.H.; Krois, J.; Uribe, S.E.; Mahmoudinia, E.; Rokhshad, R.; Nadimi, M.; Schwendicke, F. Deep Learning for Caries Detection: A Systematic Review. J. Dent. 2022, 122, 104115. [Google Scholar] [CrossRef] [PubMed]
- Ezhov, M.; Gusarev, M.; Golitsyna, M.; Yates, J.M.; Kushnerev, E.; Tamimi, D.; Aksoy, S.; Shumilov, E.; Sanders, A.; Orhan, K. Clinically Applicable Artificial Intelligence System for Dental Diagnosis with CBCT. Sci. Rep. 2021, 11, 15006. [Google Scholar] [CrossRef]
- Venskutonis, T.; Plotino, G.; Juodzbalys, G.; Mickevičiene, L. The Importance of Cone-Beam Computed Tomography in the Management of Endodontic Problems: A Review of the Literature. J. Endod. 2014, 40, 1895–1901. [Google Scholar] [CrossRef]

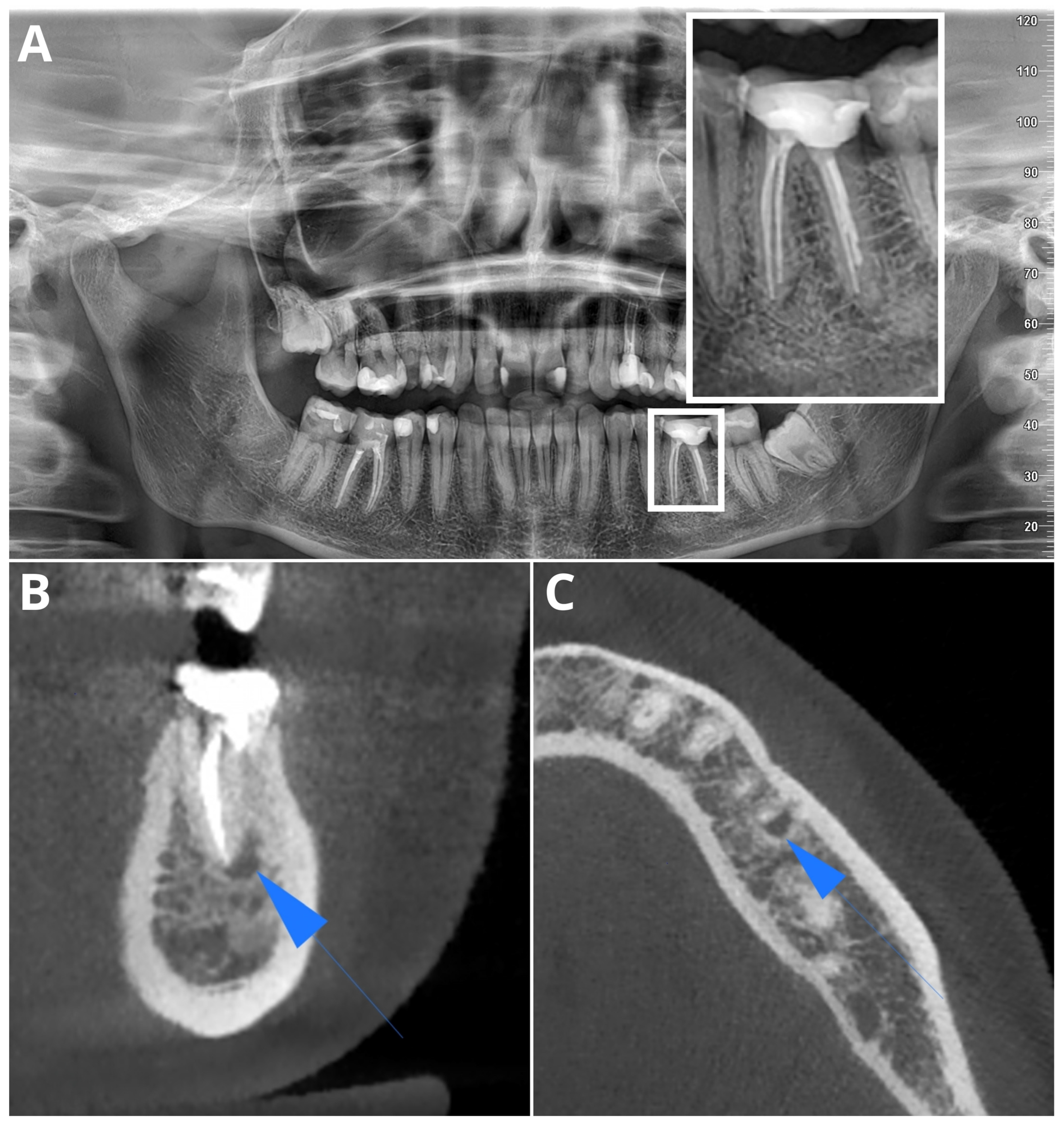
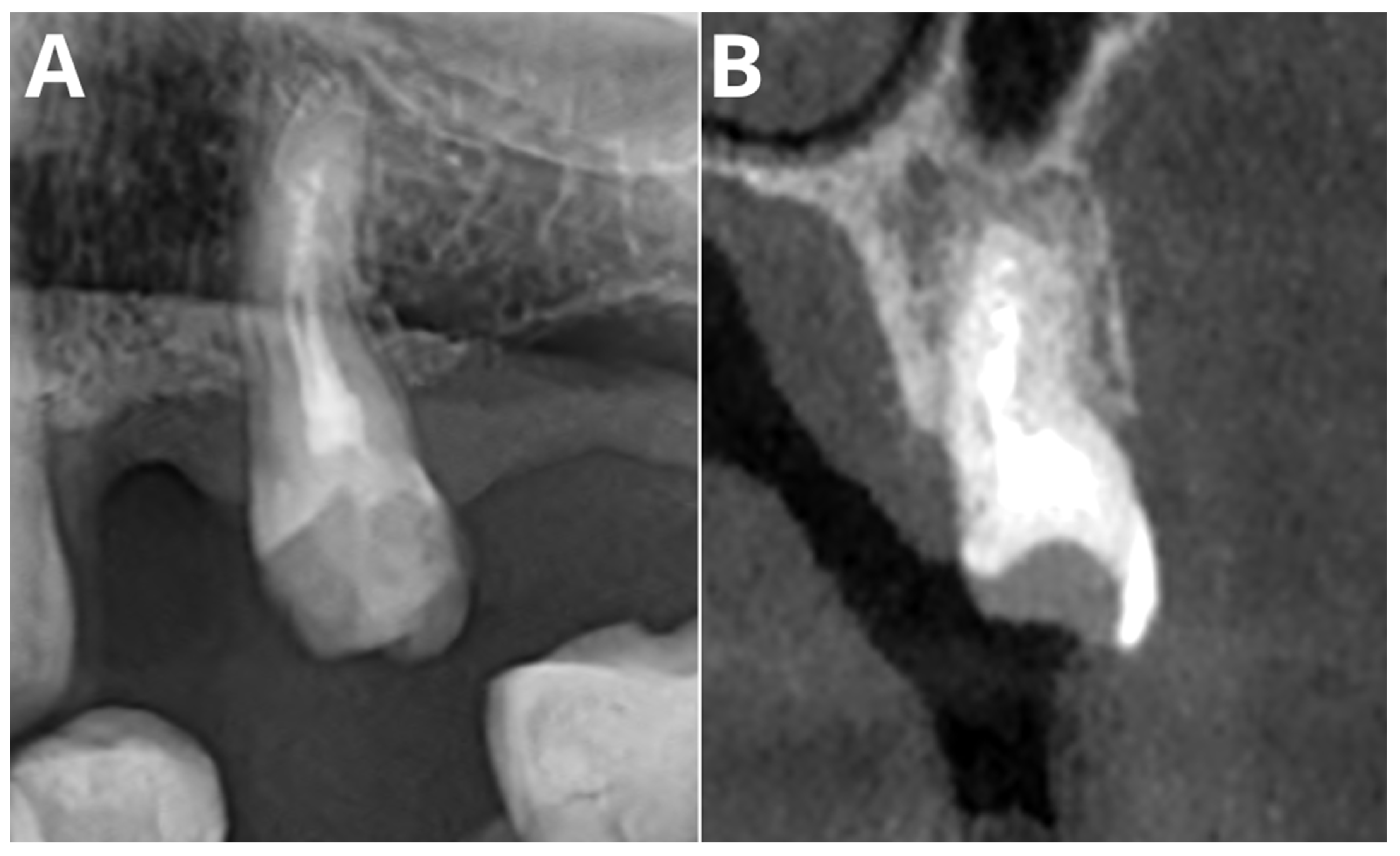

| Parameter | AI Tool | Readers’ Consensus | ||
|---|---|---|---|---|
| OPG | CBCT | OPG | CBCT | |
| PL | 28 | 23 | 51 | 26 |
| Modality | Sensitivity | Specificity | Accuracy | PPV | NPV | F1 |
|---|---|---|---|---|---|---|
| OPG | 33.33% | 98.43% | 97.01% | 32.14% | 98.51% | 32.73% |
| CBCT | 77.78% | 99.83% | 99.35% | 91.30% | 99.50% | 84.00% |
| Sensitivity | Specificity | Accuracy | PPV | NPV | F1 |
|---|---|---|---|---|---|
| 66.67% | 96.82% | 96.18% | 31.58% | 99.25% | 42.86% |
| Sensitivity | Specificity | Accuracy | PPV | NPV | F1 |
|---|---|---|---|---|---|
| 21.05% | 98.66% | 95.13% | 42.86% | 96.33% | 28.24% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, W.; Wajer, R.; Wajer, A.; Kiian, V.; Kloska, A.; Kazimierczak, N.; Janiszewska-Olszowska, J.; Serafin, Z. Periapical Lesions in Panoramic Radiography and CBCT Imaging—Assessment of AI’s Diagnostic Accuracy. J. Clin. Med. 2024, 13, 2709. https://doi.org/10.3390/jcm13092709
Kazimierczak W, Wajer R, Wajer A, Kiian V, Kloska A, Kazimierczak N, Janiszewska-Olszowska J, Serafin Z. Periapical Lesions in Panoramic Radiography and CBCT Imaging—Assessment of AI’s Diagnostic Accuracy. Journal of Clinical Medicine. 2024; 13(9):2709. https://doi.org/10.3390/jcm13092709
Chicago/Turabian StyleKazimierczak, Wojciech, Róża Wajer, Adrian Wajer, Veronica Kiian, Anna Kloska, Natalia Kazimierczak, Joanna Janiszewska-Olszowska, and Zbigniew Serafin. 2024. "Periapical Lesions in Panoramic Radiography and CBCT Imaging—Assessment of AI’s Diagnostic Accuracy" Journal of Clinical Medicine 13, no. 9: 2709. https://doi.org/10.3390/jcm13092709








