Study on Microstructure and Tribological Mechanism of Mo Incorporated (AlCrTiZr)N High-Entropy Ceramics Coatings Prepared by Magnetron Sputtering
Abstract
:1. Introduction
2. Experimental
2.1. Deposition of Coatings
2.2. Microstructure Characterization
2.3. Mechanical and Tribological Tests
3. Results and Discussion
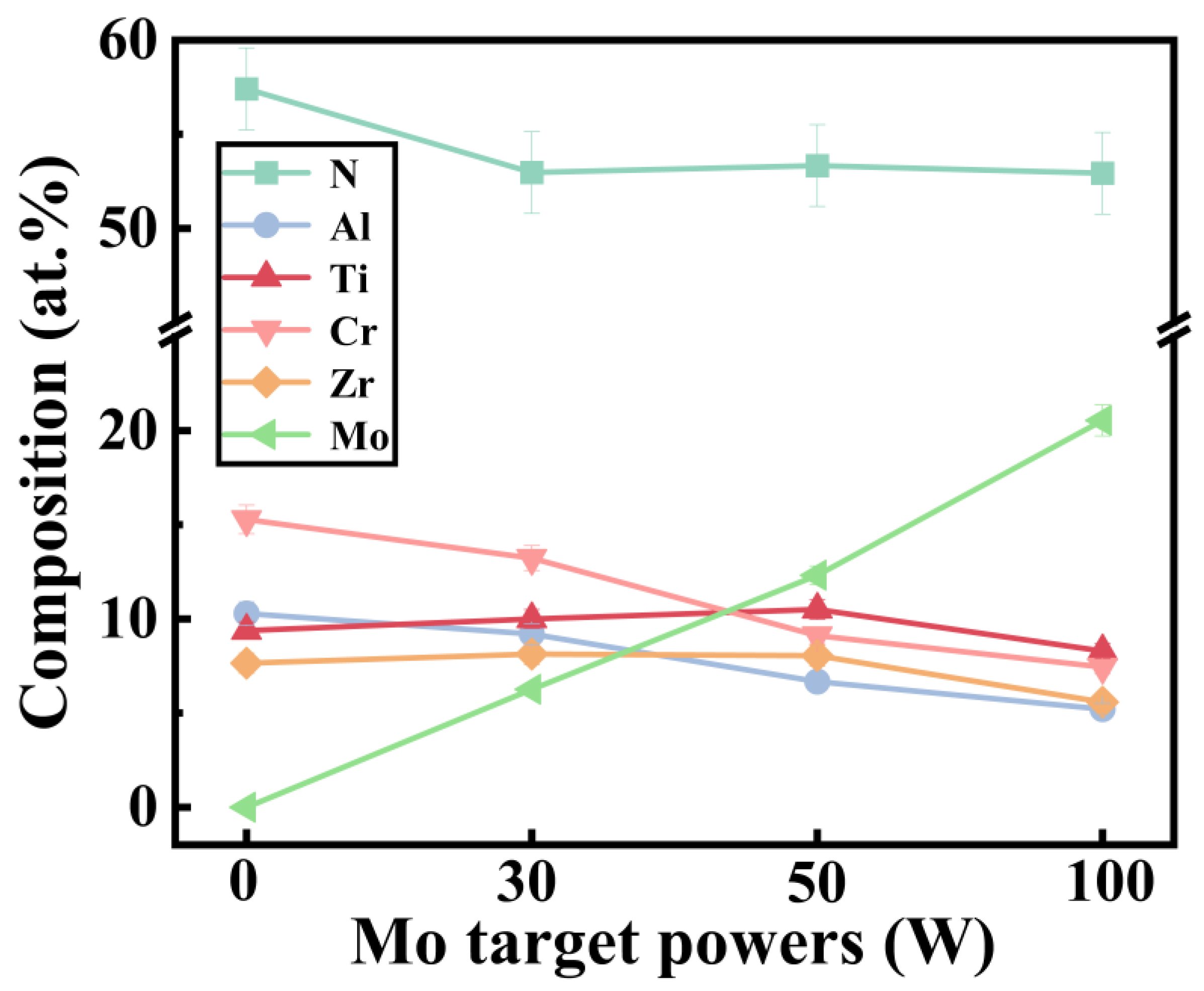
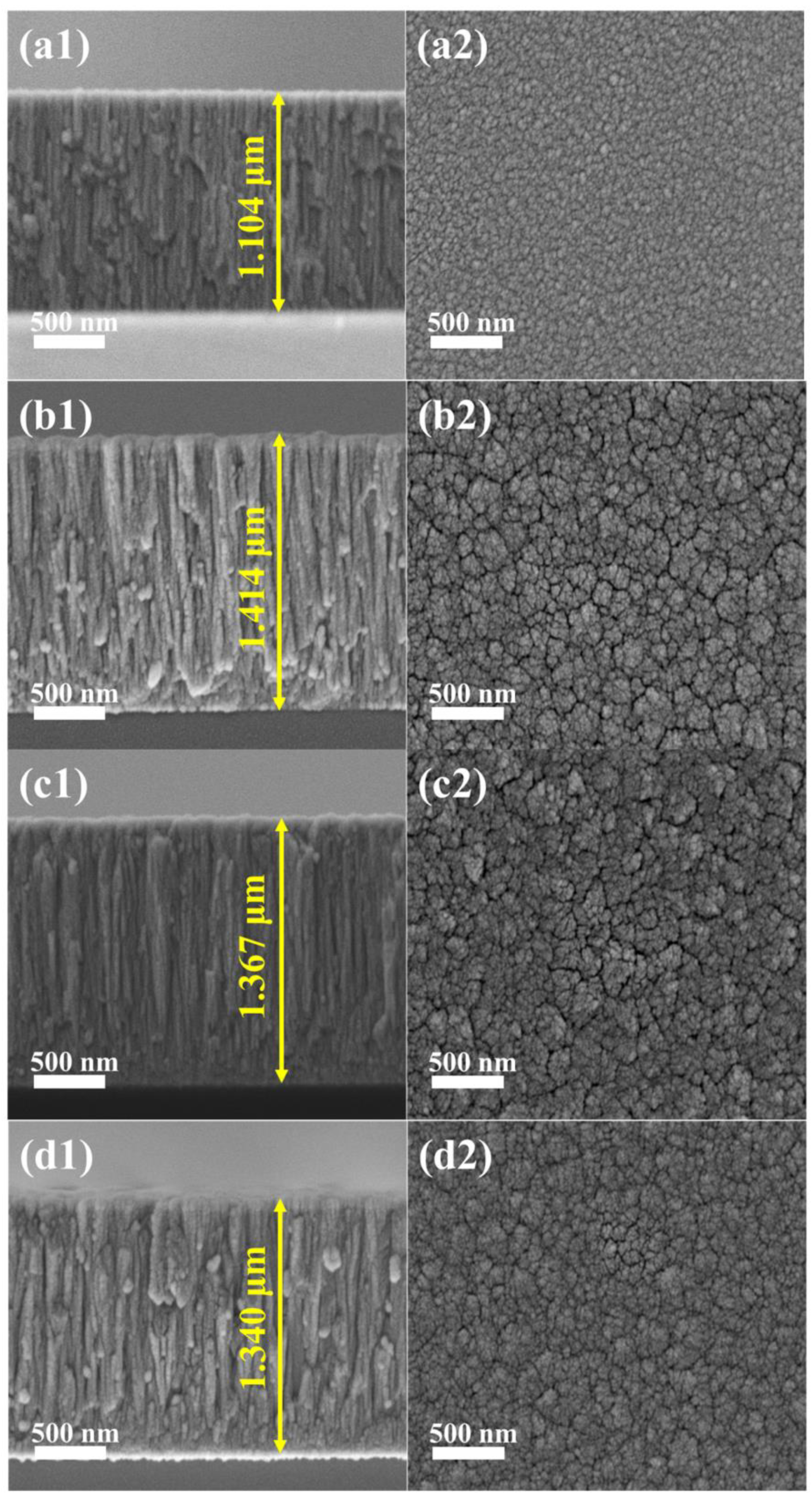
3.1. Microstructures
3.2. Mechanical Properties
3.3. Friction and Wear Properties
3.3.1. Friction Coefficient and Wear Rate
3.3.2. Wear Track Morphologies
4. Tribological Mechanism
4.1. Element Analysis of Wear Track
4.2. Wear Mechanism
5. Conclusions
- GIXRD results show that the prepared coatings are all solid solution structures. With the addition of the Mo element, the crystallinity increases and the preferred orientation of the coating changes from (111) to (200). In addition, the coating with the (111) preferred orientation presents higher roughness.
- Hard yet tough high-entropy ceramics coatings (TiZrAlCrMox)N can be obtained by magnetron sputtering, with a hardness of 30.24 GPa, and toughness (no obvious propagation cracks appear).
- The coatings with Mo show excellent tribological properties compared to those without Mo: the friction coefficient reduces to 1/3 (from 0.72 to 0.26) and the wear rate reduces to 1/10 (from 3.39 × 10−5 to 3.15 × 10−6 mm3/(N·m)).
- The low friction coefficient results from the formation of layered MoO2 which reduces the shear force, and the low wear rate results from both the high hardness and toughness.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stewart, S.; Ahmed, R. Rolling contact fatigue of surface coatings—A review. Wear 2002, 253, 1132–1144. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, S.B.; Wu, X.; Na, J.; Fung, R.F. Experimental investigation on double-impulse phenomenon of hybrid ceramic ball bearing with outer race spall. Mech. Syst. Signal Process. 2018, 113, 189–198. [Google Scholar] [CrossRef]
- Holmberg, K.; Kivikytö Reponen, P.; Härkisaari, P.; Valtonen, K.; Erdemir, A. Global energy consumption due to friction and wear in the mining industry. Tribol. Int. 2017, 115, 116–139. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Zhang, Y.; Yang, H.; Lv, G.; Gao, Y. Research progress of improving surface friction properties by surface texture technology. Int. J. Adv. Manuf. Technol. 2021, 116, 2797–2821. [Google Scholar] [CrossRef]
- Duan, W.; Sun, Y.; Liu, C.; Liu, S.; Li, Y.; Ding, C.; Ran, G.; Yu, L. Study on the formation mechanism of the glaze film formed on Ni/Ag composites. Tribol. Int. 2016, 95, 324–332. [Google Scholar] [CrossRef]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Podgornik, B.; Leskovsek, V. Wear mechanisms and surface engineering of forming tools. Mater. Tehnol. 2015, 49, 313–324. [Google Scholar] [CrossRef]
- Wu, G.; Chan, K.C.; Zhu, L.; Sun, L.; Lu, J. Dual-phase nanostructuring as a route to high-strength magnesium alloys. Nature 2017, 545, 80–83. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Lu, W.; Bao, Y.; Xia, W.; Wu, X.; Zhao, H.; Gault, B.; Liu, C.; Herbig, M.; et al. Reactive wear protection through strong and deformable oxide nanocomposite surfaces. Nat. Commun. 2021, 12, 5518. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Li, J.; Huang, J.; Kong, J.; Wu, Q.; Xiong, D. Effects of Hf addition on the structure, mechanical and tribological properties of CrN film. Surf. Coat. Technol. 2020, 397, 126067. [Google Scholar] [CrossRef]
- Banerjee, T.; Chattopadhyay, A.K. Influence of substrate bias on structural and tribo-mechanical properties of pulsed magnetron sputtered TiN-WSx hard-lubricious coating. Tribol. Int. 2018, 123, 81–91. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Ma, Q.; Callisti, M.; Polcar, T.; Yan, J. Fracture toughness and sliding properties of magnetron sputtered CrBC and CrBCN coatings. Appl. Surf. Sci. 2018, 443, 635–643. [Google Scholar] [CrossRef]
- Fan, J.; Liu, X.; Pu, J.; Shi, Y. Anti-friction mechanism of VAlTiCrMo high-entropy alloy coatings through tribo-oxidation inducing layered oxidic surface. Tribol. Int. 2022, 171, 107523. [Google Scholar] [CrossRef]
- Lo, W.L.; Hsu, S.Y.; Lin, Y.C.; Tsai, S.Y.; Lai, Y.T.; Duh, J.G. Improvement of high entropy alloy nitride coatings (AlCrNbSiTiMo)N on mechanical and high temperature tribological properties by tuning substrate bias. Surf. Coat. Technol. 2020, 401, 126247. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Liaw, P.K. Microstructures and properties of high-entropy alloy films and coatings: A review. Mater. Res. Lett. 2018, 6, 199–229. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Yakushchenko, I.V.; Beresnev, V.M. The structure and properties of high-entropy alloys and nitride coatings based on them. Russ. Chem. Rev. 2014, 83, 1027. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, S.J.; Yeh, J.W.; Chang, S.Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, C.; Li, J.; Chen, J.; Dong, Y.; Zhang, S.; Zhang, J.; Sun, D. (AlCrNiTiZr)Nx high-entropy nitride coatings with enhanced hardness via tailoring N2 flow rates for anti-wear applications. J. Vac. Sci. Technol. A 2023, 41, 053404. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; dos Santos, C.M.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. A 2016, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.H.; Zhen, J.M.; Zhao, X.C.; Ma, J.; Chen, H.; Guo, S.; Wang, C.G.; Wang, C.Z. Effect of Sn addition on the tribological behaviors of CoCrFeNi high entropy alloys. J. Alloys Compd. 2022, 909, 164657. [Google Scholar] [CrossRef]
- Gong, K.; Luo, H.; Feng, D.; Li, C. Wear of Ni3Al-based materials and its chromium-carbide reinforced composites. Wear 2008, 265, 1751–1755. [Google Scholar] [CrossRef]
- Cheng, K.H.; Lai, C.H.; Lin, S.J.; Yeh, J.W. Structural and mechanical properties of multi-element (AlCrMoTaTiZr)Nx coatings by reactive magnetron sputtering. Thin Solid Films 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Chang, H.W.; Huang, P.K.; Davison, A.; Yeh, J.W.; Tsau, C.H.; Yang, C.C. Nitride films deposited from an equimolar Al-Cr-Mo-Si-Ti alloy target by reactive direct current magnetron sputtering. Thin Solid Films 2008, 516, 6402–6408. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhao, Y.; Shi, X.; Wang, S.; Zhang, S. Super-hard (MoSiTiVZr)Nx high-entropy nitride coatings. J. Alloys Compd. 2022, 926, 166807. [Google Scholar] [CrossRef]
- Pelleg, J.; Zevin, L.Z.; Lungo, S.; Croitoru, N. Reactive-sputter-deposited TiN films on glass substrates. Thin Solid Films 1991, 197, 117–128. [Google Scholar] [CrossRef]
- Lai, C.H.; Cheng, K.H.; Lin, S.J.; Yeh, J.W. Mechanical and tribological properties of multi-element (AlCrTaTiZr)N coatings. Surf. Coat. Technol. 2008, 202, 3732–3738. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, C.; Sui, X.; Lu, X.; Zhang, X.; Wang, C.; Hao, J.; Shi, Z. Microstructure, mechanical properties and tribo-corrosion mechanism of (CrNbTiAlVMo)xN1−x coated 316 L stainless steel in 3.5 wt% NaCl solution. Tribol. Int. 2022, 173, 107638. [Google Scholar] [CrossRef]
- El Garah, M.; Touaibia, D.E.; Achache, S.; Michau, A.; Sviridova, E.; Postnikov, P.S.; Chehimi, M.M.; Schuster, F.; Sanchette, F. Effect of nitrogen content on structural and mechanical properties of AlTiZrTaHf(N) high entropy films deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2022, 432, 128051. [Google Scholar] [CrossRef]
- Greczynski, G.; Primetzhofer, D.; Lu, J.; Hultman, L. Core-level spectra and binding energies of transition metal nitrides by non-destructive X-ray photoelectron spectroscopy through capping layers. Appl. Surf. Sci. 2017, 396, 347–358. [Google Scholar] [CrossRef]
- Kubo, Y.; Sonohara, Y.; Uemura, S. Changes in the chemical state of metallic Cr during deposition on a polyimide substrate: Full soft XPS and ToF-SIMS depth profiles. Appl. Surf. Sci. 2021, 553, 149437. [Google Scholar] [CrossRef]
- Laszczynska, A.; Tylus, W.; Winiarski, J.; Szczygiel, I. Evolution of corrosion resistance and passive film properties of Ni-Mo alloy coatings during exposure to 0.5 M NaCl solution. Surf. Coat. Technol. 2017, 317, 26–37. [Google Scholar] [CrossRef]
- Kia, A.M.; Speulmanns, J.; Bönhardt, S.; Emara, J.; Kühnel, K.; Haufe, N.; Weinreich, W. Spectroscopic analysis of ultra-thin TiN as a diffusion barrier for lithium-ion batteries by ToF-SIMS, XPS, and EELS. Appl. Surf. Sci. 2021, 564, 150457. [Google Scholar] [CrossRef]
- Pshyk, A.V.; Vasylenko, A.; Bakhit, B.; Hultman, L.; Schweizer, P.; Edwards, T.E.J.; Michler, J.; Greczynski, G. High-entropy transition metal nitride thin films alloyed with Al: Microstructure, phase composition and mechanical properties. Mater. Des. 2022, 219, 110798. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, K.; Wei, S.; Zhang, H.; Yang, S.; Huang, X.; Lin, S.; Plucknett, K.P.; Lin, H. Reaction mechanisms of nano-sized AlN powders synthesized from dicyandiamide and its optical property. Mater. Chem. Phys. 2020, 253, 123376. [Google Scholar] [CrossRef]
- Kim, G.T.; Park, T.K.; Chung, H.; Kim, Y.T.; Kwon, M.H.; Choi, J.G. Growth and characterization of chloronitroaniline crystals for optical parametric oscillators: I. XPS study of Mo-based compounds. Appl. Surf. Sci. 1999, 152, 35–43. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, F.; Wang, Q.; Fu, Y.; Zhou, Z. Structural and tribological properties of CrMoCN coatings with various Mo contents in artificial seawater. Appl. Surf. Sci. 2019, 493, 485–496. [Google Scholar] [CrossRef]
- Karabacak, T.; Wang, G.C.; Lu, T.M. Physical self-assembly and the nucleation of three-dimensional nanostructures by oblique angle deposition. J. Vac. Sci. Technol. A 2004, 22, 1778–1784. [Google Scholar] [CrossRef]
- Chen, R.; Cai, Z.; Pu, J.; Lu, Z.; Chen, S.; Zheng, S.; Zeng, C. Effects of nitriding on the microstructure and properties of VAlTiCrMo high-entropy alloy coatings by sputtering technique. J. Alloys Compd. 2020, 827, 153836. [Google Scholar] [CrossRef]
- Cui, P.; Li, W.; Liu, P.; Zhang, K.; Ma, F.; Chen, X.; Feng, R.; Liaw, P.K. Effects of nitrogen content on microstructures and mechanical properties of (AlCrTiZrHf)N high-entropy alloy nitride films. J. Alloys Compd. 2020, 834, 155063. [Google Scholar] [CrossRef]
- Liang, S.C.; Chang, Z.C.; Tsai, D.C.; Lin, Y.C.; Sung, H.S.; Deng, M.J.; Shieu, F.S. Effects of substrate temperature on the structure and mechanical properties of (TiVCrZrHf)N coatings. Appl. Surf. Sci. 2011, 257, 7709–7713. [Google Scholar] [CrossRef]
- Alema, F.; Ledyaev, O.; Miller, R.; Beletsky, V.; Osinsky, A.; Schoenfeld, W.V. Growth of high Mg content wurtzite MgZnO epitaxial films via pulsed metal organic chemical vapor deposition. J. Cryst. Growth 2016, 435, 6–11. [Google Scholar] [CrossRef]
- Wan, Q.; Jia, B.Y.; Liu, P.; Luo, Y.; Chen, J.; Zhang, X.Y.; Xiao, Y.Y.; Abdelkader, T.K.; Refai, M.; Zhang, J.; et al. Microstructure and mechanical properties of FeCoCrNiAl0.1N high entropy alloy nitride coatings synthesized by cathodic arc ion plating using alloy target. Surf. Coat. Technol. 2023, 457, 129305. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef]
- Jiao, Q.; Guo, F.; Li, C.; Zheng, G.; He, J.; Zhao, H.; Qin, Y.; Yin, F. Effects of Mo addition on tribological performance of plasma-sprayed Ti-Si-C coatings. Ceram. Int. 2020, 46, 12948–12954. [Google Scholar] [CrossRef]
- Ju, H.; Ding, N.; Xu, J.; Yu, L.; Geng, Y.; Ahmed, F.; Zuo, B.; Shao, L. The influence of crystal structure and the enhancement of mechanical and frictional properties of titanium nitride film by addition of ruthenium. Appl. Surf. Sci. 2019, 489, 247–254. [Google Scholar] [CrossRef]
- Bhowmick, S.; Bhide, R.; Hoffman, M.; Jayaram, V.; Biswas, S.K. Fracture mode transitions during indentation of columnar TiN coatings on metal. Philos. Mag. 2005, 85, 2927–2945. [Google Scholar] [CrossRef]
- Wang, J.; Kuang, S.; Yu, X.; Wang, L.; Huang, W. Tribo-mechanical properties of CrNbTiMoZr high-entropy alloy film synthesized by direct current magnetron sputtering. Surf. Coat. Technol. 2020, 403, 126374. [Google Scholar] [CrossRef]











| DC Power of Target (W) | Deposition Time | RF Bias Power (W) | N2 Flow Rate (sccm) | Ar Flow Rate (sccm) | ||
|---|---|---|---|---|---|---|
| TiZr 200 | AlCr 150 | Mo 0/30/50/100 | 240/230 /210/190 | 30 | 12 | 40 |
| Mo Content | d (nm) | a (Å) | H (GPa) | E (GPa) | H/E | H3/E2 (GPa) | μ | Wr (mm3/(N•m)) |
|---|---|---|---|---|---|---|---|---|
| 0 at.% | 6.5 | 4.2849 | 24.39 | 317.91 | 0.077 | 0.144 | 0.72 | 3.39 × 10−5 |
| 6.26 at.% | 7.1 | 4.3022 | 24.44 | 303.56 | 0.081 | 0.158 | 0.35 | 5.78 × 10−6 |
| 12.32 at.% | 6.3 | 4.3138 | 30.24 | 327.83 | 0.092 | 0.257 | 0.26 | 3.15 × 10−6 |
| 20.54 at.% | 5.8 | 4.3010 | 26.41 | 315.76 | 0.084 | 0.185 | 0.38 | 6.18 × 10−6 |
| (TiZrAlCrMox)N | Ti (at.%) | Zr (at.%) | Al (at.%) | Cr (at.%) | Mo (at.%) | |
|---|---|---|---|---|---|---|
| Ti-N-O | Ti-O | Zr-O | Al-O | Cr-O | Mo-O | |
| 0 at.% Mo-pre | 22.64 | 16.15 | 53.1 | 46.34 | 45.73 | |
| 0 at.% Mo-post | 31.74 | 18.56 | 45.48 | 55.48 | 53.23 | |
| 12.32 at.% Mo-pre | 19.78 | 18.49 | 50.01 | 39.84 | 50.93 | 28.10 |
| 12.32 at.% Mo-post | 28.48 | 21.35 | 49.99 | 67.45 | 57.39 | 41.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Zhao, Y.; Li, J.; Zhang, S.; Zhang, J.; Sun, D. Study on Microstructure and Tribological Mechanism of Mo Incorporated (AlCrTiZr)N High-Entropy Ceramics Coatings Prepared by Magnetron Sputtering. Nanomaterials 2024, 14, 814. https://doi.org/10.3390/nano14100814
Zheng J, Zhao Y, Li J, Zhang S, Zhang J, Sun D. Study on Microstructure and Tribological Mechanism of Mo Incorporated (AlCrTiZr)N High-Entropy Ceramics Coatings Prepared by Magnetron Sputtering. Nanomaterials. 2024; 14(10):814. https://doi.org/10.3390/nano14100814
Chicago/Turabian StyleZheng, Jia, Yiman Zhao, Jingchuan Li, Sam Zhang, Jian Zhang, and Deen Sun. 2024. "Study on Microstructure and Tribological Mechanism of Mo Incorporated (AlCrTiZr)N High-Entropy Ceramics Coatings Prepared by Magnetron Sputtering" Nanomaterials 14, no. 10: 814. https://doi.org/10.3390/nano14100814





