Exploring the Synergistic Mechanisms of Nanopulsed Plasma Bubbles and Photocatalysts for Trimethoprim Degradation and Mineralization in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup, Electrical Diagnostics and Treatment Conditions
2.3. Chemical Analysis of Water Samples
2.4. Identification of Reactive Species in Gas and Aqueous Phase and Use of Scavengers
2.5. Catalyst Characterization
3. Results
3.1. TiO2 and ZnO Characterization (XRD, BET) before and after Plasma Treatment and TEM Images
3.2. Electrical and Optical Plasma Characterization
3.3. Effect of Catalyst Addition on Plasma-Treated Water Characteristics
3.3.1. Plasma Species Formation
3.3.2. Physicochemical Water Properties
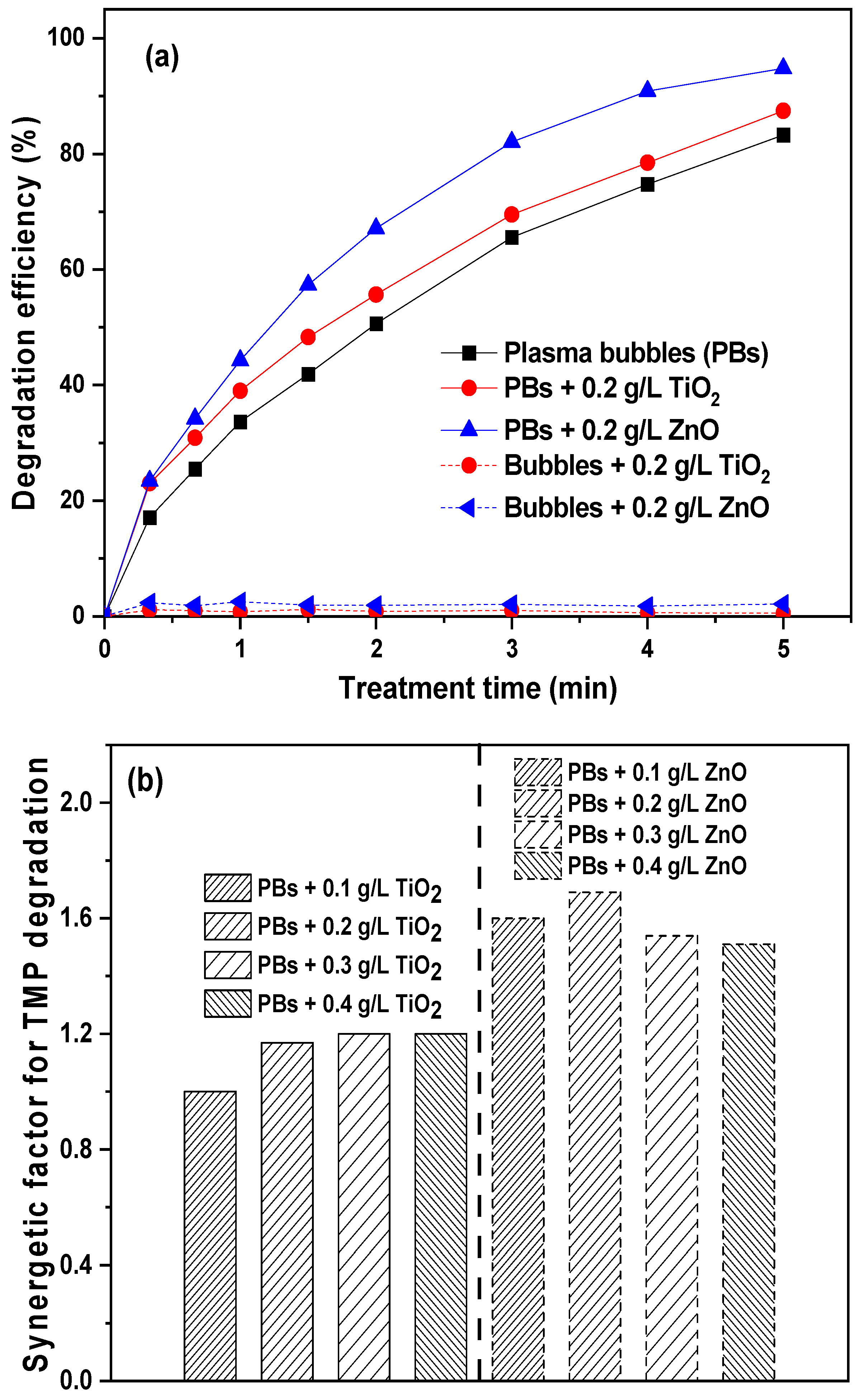
3.4. The Effect of Catalyst Loading on the Degradation of TMP
3.5. The Effect of Plasma Gas and Initial TMP Concentration on Its Degradation by Plasma Bubbles in the Absence and Presence of ZnO
3.6. Process Energy Efficiency
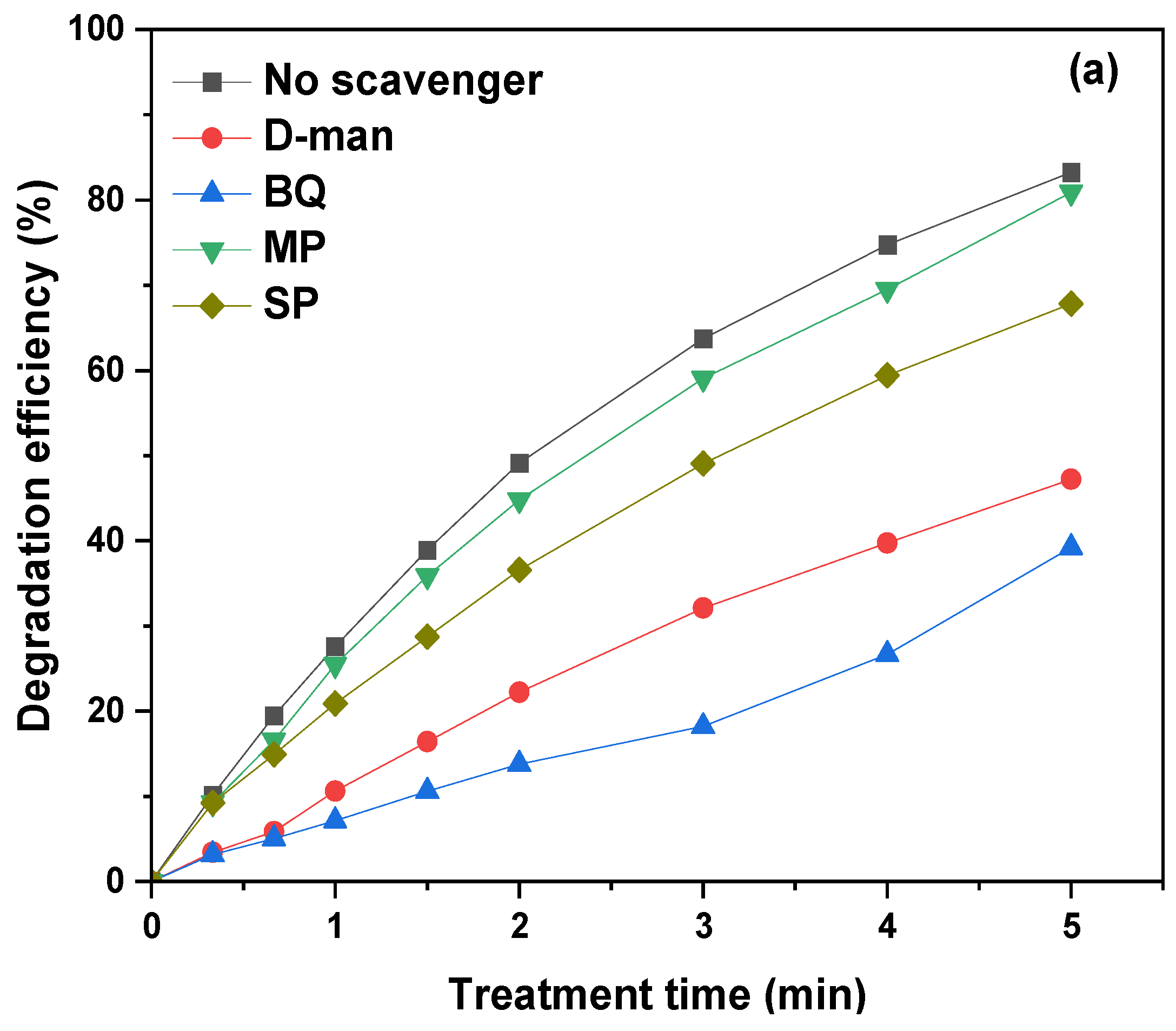
3.7. The Role of Plasma Species in the Degradation of TMP by Plasma Bubbles
3.8. The Mineralization of TMP under Plasma Bubbles in the Absence and Presence of ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Musie, W.; Gonfa, G. Fresh water resource, scarcity, water salinity challenges and possible remedies: A review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef] [PubMed]
- Syeed, M.M.; Hossain, M.S.; Karim, M.R.; Uddin, M.F.; Hasan, M.; Khan, R.H. Surface water quality profiling using the water quality index, pollution index and statistical methods: A critical review. Environ. Sustain. Indic. 2023, 18, 100247. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N., Jr.; Machado, S.A.S. Paper-based electrochemical sensors with reduced graphene nanoribbons for simultaneous detection of sulfamethoxazole and trimethoprim in water samples. J. Electroanal. Chem. 2021, 882, 114985. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhao, S. A new electrochemical sensor for simultaneous detection of sulfamethoxazole and trimethoprim antibiotics based on graphene and ZnO nanorods modified glassy carbon electrode. Microchem. J. 2020, 159, 105440. [Google Scholar] [CrossRef]
- Mpatani, F.M.; Aryee, A.A.; Kani, A.N.; Han, R.; Li, Z.; Dovi, E.; Qu, L. A review of treatment techniques applied for selective removal of emerging pollutant-trimethoprim from aqueous systems. J. Clean. Prod. 2021, 308, 127359. [Google Scholar] [CrossRef]
- Dolar, D.; Vuković, A.; Ašperger, D.; Košutić, K. Efficiency of RO/NF membranes at the removal of veterinary antibiotics. Water Sci. Technol. 2012, 65, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Kyere-Yeboah, K.; Bique, I.K.; Qiao, X.-c. Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants. A comprehensive review. Chemosphere 2023, 320, 138061. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, C.A. Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Aggelopoulos, C.; Meropoulis, S.; Hatzisymeon, M.; Lada, Z.G.; Rassias, G. Degradation of antibiotic enrofloxacin in water by gas-liquid nsp-DBD plasma: Parametric analysis, effect of H2O2 and CaO2 additives and exploration of degradation mechanisms. Chem. Eng. J. 2020, 398, 125622. [Google Scholar] [CrossRef]
- Gao, X.; Huang, K.; Zhang, A.; Wang, C.; Sun, Z.; Liu, Y. Simultaneous degradation of glucocorticoids and sterilization using bubbling corona discharge plasma based systems: A promising terminal water treatment facility for hospital wastewater. Chem. Eng. J. 2021, 430, 132845. [Google Scholar] [CrossRef] [PubMed]
- Magureanu, M.; Bilea, F.; Bradu, C.; Hong, D. A review on non-thermal plasma treatment of water contaminated with antibiotics. J. Hazard. Mater. 2021, 417, 125481. [Google Scholar] [CrossRef] [PubMed]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Meropoulis, S.; Giannoulia, S.; Skandalis, S.; Rassias, G.; Aggelopoulos, C.A. Key-study on plasma-induced degradation of cephalosporins in water: Process optimization, assessment of degradation mechanisms and residual toxicity. Sep. Purif. Technol. 2022, 298, 121639. [Google Scholar] [CrossRef]
- Schneider, M.; Rataj, R.; Bláha, L.; Kolb, J.F. Experimental review of different plasma technologies for the degradation of cylindrospermopsin as model water pollutant. Chem. Eng. J. 2023, 451, 138984. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, R.; Wang, P.; Mai-Prochnow, A.; McConchie, R.; Li, W.; Zhou, R.; Thompson, E.W.; Ostrikov, K.; Cullen, P.J. Degradation of cefixime antibiotic in water by atmospheric plasma bubbles: Performance, degradation pathways and toxicity evaluation. Chem. Eng. J. 2021, 421, 127730. [Google Scholar] [CrossRef]
- Meropoulis, S.; Aggelopoulos, C.A. Plasma microbubbles vs. gas-liquid DBD energized by low-frequency high voltage nanopulses for pollutants degradation in water: Destruction mechanisms, composition of plasma-activated water and energy assessment. J. Environ. Chem. Eng. 2023, 11, 109855. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Alam, D.; Zhang, T.; Li, W.; Xia, Y.; Mai-Prochnow, A.; An, H.; Lovell, E.C.; Masood, H.; et al. Plasmacatalytic bubbles using CeO2 for organic pollutant degradation. Chem. Eng. J. 2021, 403, 126413. [Google Scholar] [CrossRef]
- Ansari, M.; Sharifian, M.; Ehrampoush, M.H.; Mahvi, A.H.; Salmani, M.H.; Fallahzadeh, H. Dielectric barrier discharge plasma with photocatalysts as a hybrid emerging technology for degradation of synthetic organic compounds in aqueous environments: A critical review. Chemosphere 2021, 263, 128065. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, C.A.; Dolinski, O. A comprehensive insight on plasma-catalytic degradation of organic pollutants in water: Comparison between ZnO and TiO2. Chemosphere 2024, 347, 140667. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Yang, C.-S.; Mok, Y.S. Degradation of veterinary antibiotics by dielectric barrier discharge plasma. Chem. Eng. J. 2013, 219, 19–27. [Google Scholar] [CrossRef]
- Liang, J.-P.; Zhou, X.-F.; Zhao, Z.-L.; Yang, D.-Z.; Wang, W.-C. Degradation of trimethoprim in aqueous by persulfate activated with nanosecond pulsed gas-liquid discharge plasma. J. Environ. Manag. 2021, 278, 111539. [Google Scholar] [CrossRef] [PubMed]
- Hatzisymeon, M.; Daletou, M.K.; Rassias, G.; Aggelopoulos, C.A. Degradation of organic pollutants combining plasma discharges generated within soil with TiO2 and ZnO catalysts: Comparative analysis, optimization and mechanisms. Sep. Purif. Technol. 2023, 320, 124119. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, T.; Zhou, R.; Zhou, R.; Ostikov, K.; Rezaeimotlagh, A.; Cullen, P.J. Plasma bubbles: A route to sustainable chemistry. AAPPS Bull. 2021, 31, 26. [Google Scholar] [CrossRef]
- Li, S.; Timoshkin, I.V.; Maclean, M.; Macgregor, S.J.; Wilson, M.P.; Given, M.J.; Wang, T.; Anderson, J.G. Fluorescence detection of hydroxyl radicals in water produced by atmospheric pulsed discharges. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1856–1865. [Google Scholar] [CrossRef]
- O’Sullivan, D.W.; Tyree, M. The kinetics of complex formation between Ti(IV) and hydrogen peroxide. Int. J. Chem. Kinet. 2007, 39, 457–461. [Google Scholar] [CrossRef]
- Jamróz, P.; Pohl, P.; Żyrnicki, W. Spectroscopic evaluation of a low power atmospheric pressure mixed argon–helium microwave induced plasma combined with the chemical generation of volatile species for the optical emission spectrometric determination of arsenic, antimony and mercury. J. Anal. At. Spectrom. 2012, 27, 1772–1779. [Google Scholar] [CrossRef]
- Rezaei, F.; Abbasi-Firouzjah, M.; Shokri, B. Investigation of antibacterial and wettability behaviours of plasma-modified PMMA films for application in ophthalmology. J. Phys. D Appl. Phys. 2014, 47, 085401. [Google Scholar] [CrossRef]
- Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Plasma Modification of Poly Lactic Acid Solutions to Generate High Quality Electrospun PLA Nanofibers. Sci. Rep. 2018, 8, 2241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Wang, P.; Luan, B.; Zhang, X.; Fang, Z.; Xian, Y.; Lu, X.; Ostrikov, K.K.; Bazaka, K. Microplasma Bubbles: Reactive Vehicles for Biofilm Dispersal. ACS Appl. Mater. Interfaces 2019, 11, 20660–20669. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Shen, Z.; Huang, Y.; Lu, J.; Ren, D.; Sun, J.; Cao, J.; Liu, H. Post-plasma-catalytic removal of toluene using MnO2–Co3O4 catalysts and their synergistic mechanism. Chem. Eng. J. 2018, 348, 15–25. [Google Scholar] [CrossRef]
- Wu, K.; Sun, Y.; Liu, J.; Xiong, J.; Wu, J.; Zhang, J.; Fu, M.; Chen, L.; Huang, H.; Ye, D. Nonthermal plasma catalysis for toluene decomposition over BaTiO3-based catalysts by Ce doping at A-sites: The role of surface-reactive oxygen species. J. Hazard. Mater. 2021, 405, 124156. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, S.; Dang, X.; Wang, H.; Qu, J.; Zheng, H. Facile fabrication of three-dimensional MnO2 for trichloroethylene degradation by plasma catalysis. Sep. Purif. Technol. 2023, 325, 124680. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Huang, C.P.; Dong, C.; Tang, Z. Advanced chemical oxidation: Its present role and potential future in hazardous waste treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]
- Marotta, E.; Ceriani, E.; Schiorlin, M.; Ceretta, C.; Paradisi, C. Comparison of the rates of phenol advanced oxidation in deionized and tap water within a dielectric barrier discharge reactor. Water Res. 2012, 46, 6239–6246. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, V.; Dojcinovic, B.; Jović, M.; Roglic, G.; Obradović, B.; Kuraica, M. Measurement of reactive species generated by dielectric barrier discharge in direct contact with water in different atmospheres. J. Phys. D Appl. Phys. 2017, 50, 19. [Google Scholar] [CrossRef]
- Gong, S.; Sun, Y.; Zheng, K.; Jiang, G.; Li, L.; Feng, J. Degradation of levofloxacin in aqueous solution by non-thermal plasma combined with Ag3PO4/activated carbon fibers: Mechanism and degradation pathways. Sep. Purif. Technol. 2020, 250, 117264. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, N.; Wang, H.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Pulsed discharge plasma assisted with graphene-WO3 nanocomposites for synergistic degradation of antibiotic enrofloxacin in water. Chem. Eng. J. 2019, 372, 226–240. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, N.; Wang, H.; Shang, K.; Lu, N.; Li, J.; Wu, Y. Enhanced catalytic performance of graphene-TiO2 nanocomposites for synergetic degradation of fluoroquinolone antibiotic in pulsed discharge plasma system. Appl. Catal. B Environ. 2019, 248, 552–566. [Google Scholar] [CrossRef]
- Li, Y.; Xie, W.; Hu, X.; Shen, G.; Zhou, X.; Xiang, Y.; Zhao, X.; Fang, P. Comparison of Dye Photodegradation and its Coupling with Light-to-Electricity Conversion over TiO2 and ZnO. Langmuir 2010, 26, 591–597. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, C.; Liu, Y.; Chen, H.; Li, H.; Wu, J. Comparison on photocatalytic degradation of gaseous formaldehyde by TiO2, ZnO and their composite. Ceram. Int. 2012, 38, 4437–4444. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Solar Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Chantes, P.; Jarusutthirak, C.; Danwittayakul, S. A comparison study of photocatalytic activity of TiO2 and ZnO on the degradation of real batik wastewater. In Proceedings of the International Conference on Biological, Environment and Food Engineering (BEFE-2015), Singapore, 15–16 May 2015. [Google Scholar]
- Murugesan, P.; Evanjalin Monica, V.; Moses, J.A.; Anandharamakrishnan, C. Water decontamination using non-thermal plasma: Concepts, applications, and prospects. J. Environ. Chem. Eng. 2020, 8, 104377. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Zheng, L.; Liu, Y.; Zhao, Y.; Huang, S.; Li, S. Synergistic catalysis degradation of amoxicillin by DBD plasma-catalyst system constructed by DBD plasma and Ce0.5Bi0.5VO4/HCP coating. Process Saf. Environ. Prot. 2024, 181, 416–428. [Google Scholar] [CrossRef]
- Xiao, S.; Shen, Z.; Song, S.; Han, S.; Du, Y.; Wang, H. Enhanced sulfadiazine degradation in a multi-electrode paralleling DBD plasma system coupled with ZnO/cellulose acetate films. J. Environ. Chem. Eng. 2023, 11, 109063. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yao, X.; Zhang, Y.; Li, Z.; Pan, S.; Han, J.; Xu, L.; Qiao, W.; Li, J.; et al. A comprehensive insight into plasma-catalytic removal of antibiotic oxytetracycline based on graphene-TiO2-Fe3O4 nanocomposites. Chem. Eng. J. 2021, 425, 130614. [Google Scholar] [CrossRef]
- Hu, K.; Xie, Q.; Wang, H.; Zhang, B.; Huang, Y.; Song, S.; Zhang, H.; Ding, Y.; Huang, H.; Wu, C. Synergistic catalysis of Cu-CeO2@CA composite film in a circulating DBD plasma system and its effect on ciprofloxacin degradation. Chem. Eng. J. 2023, 455, 140895. [Google Scholar] [CrossRef]
- Ye, W.-K.; Tian, F.-X.; Chen, C.; Ye, J.; Liu, F.-w.; Wang, B.; Hu, X.-J.; Xu, B. Performance evaluation of the UV activated chlorite process on trimethoprim: Degradation efficiency, energy consumption and disinfection by-products formation. Chemosphere 2023, 327, 138540. [Google Scholar] [CrossRef] [PubMed]
- Ljubas, D.; Juretić, H.; Badrov, A.; Biošić, M.; Babić, S. Photocatalytic Degradation of Pharmaceutical Trimethoprim in Aqueous Solution over Nanostructured TiO2 Film Irradiated with Simulated Solar Radiation. Appl. Sci. 2023, 13, 5681. [Google Scholar] [CrossRef]









| Specific Surface Area (m2/g)/Pore Volume (cm3/g) | ||
|---|---|---|
| Catalyst | Pristine | Plasma-Treated |
| TiO2 | 54.96/0.121 | 54.92/0.160 |
| ZnO | 13.18/0.024 | 13.01/0.026 |
| Plasma Process | Pollutant | Degradation Efficiency (%) | Treatment Time (min) | EEO (kWh/m3) | Ref. |
|---|---|---|---|---|---|
| DBD plasma + Ce0.5Bi0.5VO4 | Amoxicillin 50 mg/L | 94.5 | 30 | 79.4 | [47] |
| DBD plasma + ZnO on cellulose acetate films | Sulfadiazine 20 mg/L | 97.2 | 60 | 15.4 | [48] |
| Plasma + graphene–TiO2–Fe3O4 | Oxytetracycline 40 mg/L | 98.1 | 60 | - | [49] |
| DBD + Cu-CeO2@CA | Ciprofloxacin 200 mg/L | 89.5 | 40 | - | [50] |
| UV–chloride (1 mM) | Trimethoprim 2.9 mg/L | 91 | 20 | 3.6 | [51] |
| Photocatalysis (TiO2 film irradiated with simulated solar radiation) | Trimethoprim 10 mg/L | 90 | 101.5 | 8441.4 | [52] |
| Nanopulsed air–plasma bubbles + ZnO | Trimethoprim 20 mg/L | 90.9 | 4 | 0.46 | This study |
| Nanopulsed O2–plasma bubbles | Trimethoprim 20 mg/L | 94.3 | 2 | 0.31 | This study |
| Nanopulsed O2–plasma bubbles + ZnO | Trimethoprim 20 mg/L | 95.5 | 2 | 0.28 | This study |
| Nanopulsed air–plasma bubbles + ZnO | Trimethoprim 5 mg/L | 94.2 | 1.5 | 0.23 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsokanas, D.; Aggelopoulos, C.A. Exploring the Synergistic Mechanisms of Nanopulsed Plasma Bubbles and Photocatalysts for Trimethoprim Degradation and Mineralization in Water. Nanomaterials 2024, 14, 815. https://doi.org/10.3390/nano14100815
Tsokanas D, Aggelopoulos CA. Exploring the Synergistic Mechanisms of Nanopulsed Plasma Bubbles and Photocatalysts for Trimethoprim Degradation and Mineralization in Water. Nanomaterials. 2024; 14(10):815. https://doi.org/10.3390/nano14100815
Chicago/Turabian StyleTsokanas, Dimitris, and Christos A. Aggelopoulos. 2024. "Exploring the Synergistic Mechanisms of Nanopulsed Plasma Bubbles and Photocatalysts for Trimethoprim Degradation and Mineralization in Water" Nanomaterials 14, no. 10: 815. https://doi.org/10.3390/nano14100815






